Review and Synthesis |
|
Corresponding author: Javier Loidi ( javier.loidi@ehu.eus ) Academic editor: John Hunter
© 2022 Javier Loidi, Gonzalo Navarro-Sánchez, Denys Vynokurov.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Loidi J, Navarro-Sánchez G, Vynokurov D (2022) Climatic definitions of the world’s terrestrial biomes. Vegetation Classification and Survey 3: 231-271. https://doi.org/10.3897/VCS.86102
|
Abstract
Question: Is it feasible to establish a classification of large biotic units of the world related to climatic types? Study area: The world. Methods: A total of 616 localities have been selected, their climatic parameters calculated and subjected to a PCA. The climatic characterization of biomes and subbiomes has been completed after data analysis. Results: A hierarchical classification is proposed for the biotic units within four main domains: Cryocratic, Mesocratic, Xerocratic and Thermocratic, divided into 7 ecozones, 9 biomes and 20 subbiomes linked to climatically defined territories. Most of the units are intercontinental. The mountains represent an abbreviated version of the latitudinal zonation and the altitudinal belts are related to the corresponding units of the lowlands. For the bioclimatic units, a parallel classification is proposed to fit with that of the biotic units: 4 Macrobioclimates and 10 bioclimates. Furthermore, 7 ombrotypes and 7 thermotypes are recognized to frame the climatic variation within each climatic territory due to terrain ruggedness, particularly in relation to large or medium sized mountains. Conclusions: The southern hemisphere is substantially more oceanic than the northern hemisphere. This is due to the distribution of the land masses and the modifying effect they have on the flow of air and marine currents. As a result, there is one biome and one subbiome exclusively found in the northern hemisphere (6. Biome of the steppe, and 5.b Continental scrub and woodlands subbiome) and two others which are almost confined to it (2. Biome of the boreal forest, and 3. Biome of the temperate deciduous forests). The 7. Biome of the deserts and 5. Biome of the temperate aridiestival evergreen forests and shrublands occur on the western side of the continents and expand in their interior favoured by rain shadow and continentality effects.
Taxonomic reference:
Abbreviations: ITCZ = Inter Tropical Convergence Zone; NH = Northern Hemisphere; PCA = Principal Component Analysis; SH = Southern Hemisphere.
Keywords
biome, biotic unit, climate of the world, domain, ecozone, large scale vegetation units, potential natural vegetation, subbiome, terrestrial ecosystem
Introduction
Describing the world’s vegetation on a global scale in a way that reflects the factors determining its distribution is as old as geobotany itself (von
Biomes and bioclimates
A broad-scale typology of biomes has to fulfil the two following conditions:
- An easily recognizable set of features characteristic of each biome, yet overcoming regional floristic patterns so that the biome can extend across several continents and distant territories bearing different floras.
- A consistent correlation with broad climatic units to be recognizable across the world. Broad climate types are intercontinental. If biomes have to be so, then they should also be related to the climatic types. Other conditions such as substrate, disturbance regimes and human influence lead to a more local typology of biomes and align them to a description of vegetation communities on a regional scale. In our opinion, a biome is basically a zonal unit which can diversify into more regional ones by means of relevant edaphic, hydrologic and disturbance conditions.
How do we define a biome?
This section explains the conceptual framework of what could be understood by the scientific community under the term Biome. As it is an old concept (
To reach an agreeing and unifying concept, so that polysemy and progressive “babelization” upon such term could be avoided, and connecting with the tradition of origin and use of it, two drifts should be prevented in the case of biomes: the regional drift and the dynamism drift:
Regional drift
: Many attempts to describe the biomes of the world fall to the temptation of considering vegetation units that are particular to specific regions. As examples, we can mention “Evergreen Nemoral Nothofagus Forest” (
Dynamical drift
: This results from the acceptance of units that are the result of dynamic processes, often human induced, that occur under certain disturbance or management regimes, such as the “scrub and shrub biomes” of
As
- Biota: all the biological diversity that can be found within its limits (plants, animals, fungi, etc.).
- Coenoses: all the forms of assemblages of these living beings (populations, communities).
-
Sigmeta (
Loidi 2021 ): all the processes taking place in the two aforementioned components (ecosystem functioning, disturbances and dynamics, evolution, etc.). -
Geosigmeta (
Loidi 2021 ): the spatial distribution patterns occurring within the territory of the biome, which are determined by local topography such as the crest-slope-valley zonation, and the azonal ecosystems occurring within it.
We suggest to define global-scale units exclusively by climate while other relevant ecological factors such as soil fertility, hydrologic regime, natural disturbance regime, etc., can be used to define regional or successional units.
As an integrative concept to be applied at a large scale, the biome should be defined by natural features: natural biota (flora, fauna, etc.), natural ecosystems and natural landscapes. In such a way, the biome can be used as a reference for an ideal natural situation. This implies the removal of anthropic disturbances such as farming, stockbreeding, housing, etc. In other words, as most current disturbances are human induced, we need to remove them from ecosystems and establish a “theoretical” natural state.
Moreover, the inclusion of human influence in the conceptual framework of biome has the following problems:
- Modernity. Human influence is a relatively new phenomenon on earth. It began having notable impacts on terrestrial ecosystems approximately 11 Ky ago when the Neolithic age began with agriculture and cattle raising in the Fertile Crescent. Before that period, the impact of human species was scarcely higher than that of a medium sized mammal. After that, these activities expanded throughout the world at very different paces and intensities, transforming the territories in numerous ways, and reached a truly global scale only in the last few centuries. Nonetheless, human influence in terrestrial ecosystems has been enormous, and manifests in a huge complexity of ways depending on geographical conditions and on cultural variability.
- Selective character. The way and intensity in which humans have influenced terrestrial ecosystems has also been heavily influenced by the natural conditions inherent to them. This has to do with profitability or capability of extracting goods for human use in the ecosystem in question. Humans distribute pressure, and thus modifications, concentrating it upon the most fertile environments leaving the infertile landscapes much less disturbed (YODFELS [Young, Often Disturbed, Fertile Landscapes] as opposed OCBILS [Old, Climatically Buffered, Infertile Landscapes] of
Hopper 2009 ). For example, compare the contrast of human exploitation of deserts and arid lands with that of the evergreen tropical forest, or the tundra with the temperate deciduous forest area. - Changing character. Modern technology favours a homogenization of land uses in a much greater way than was done centuries ago, so that today it is possible to grow oranges in the Arctic and corn in the desert. It is only necessary to install powerful greenhouses in the first case and irrigate intensively in the second, although the necessary energy inputs increase enormously. Thus, human influence, in addition to being diverse because it has already been conditioned by the diversity of natural ecosystems, is changing with technology and population growth.
Therefore, we propose that human influence should not be considered as a defining element for biomes, as they provide a natural reference. The creation of Anthromes (
Another point is that biomes refer to zonal ecosystems. As these respond mainly to climatic conditions, the biomes will be distributed along climatic gradients. These gradients are manifested on two spatial scales: large regions and continents (geographical scale) and mountains (altitudinal scale). In the latter, a compressed climatic gradient occurs across a small territory, leading to a chain of different biomes or vegetation belts along the altitudinal gradient. Azonal ecosystems, either humid (such as wetlands), dry (such as rocky or shallow substrates), or saline (such as coastal marshes, etc.) are excluded in our classification and are assimilated to the zonal biomes of each territory. In this way, we intend to define the climatic envelope of the recognized physiognomic units and to use them to create a truly bioclimatic classification in a similar way as has been done by some authors (
Biotic units classification
Similar to other proposals to frame all the ecosystems of the world (
Biotic unit. A generic concept which encompasses all existing biota living in a terrestrial ecological and geograpical space. As indicated above, it is a large-scale container concept that includes all biotic components: biota, species assemblages, and ecological processes occurring within the ecosystems.
Biotic units are basically determined by a climatic definition. There are biotic units of four different ranks: domain, ecozone, biome and subbiome. In some other approaches, such units could be also defined by edaphic or other conditions of high relevance. A biotic unit is not defined by the taxonomic composition of its flora, i.e., it is not a biogeographic unit. A biotic unit can occur in multiple distant areas where floristic differences are substantial. As an integrative concept, biotic units should be primarily defined by natural features: natural biota (flora, fauna, etc.), natural ecosystems, natural landscapes. “Natural” means that the human influence is less apparent at the level of noticeable ecosystem modification.
Domain. This is the largest division in the biotic units. It is characterized by broad climatic conditions (temperature and aridity) manifest in the four main belts of the earth: A. Severe cold around the poles and in the high mountains – cryocratic (governed by the cold); B. Thermic seasons in the intermediate belt between the Tropics and the cold areas, one cold and another warm – mesocratic (governed by the intermediate conditions); C. An aridity belt in the subtropics where the scarcity of moisture is the main determinant factor for living beings – xerocratic (governed by aridity); D. Absence of cold and of thermic seasons between the Tropics – thermocratic (governed by the warmth).
Ecozone. Inspired by
Biome. This is the third rank within the biotic units. Biomes are determined by the physiognomy of the zonal potential natural vegetation matching with specific climatic conditions, according to the traditional use of this term (
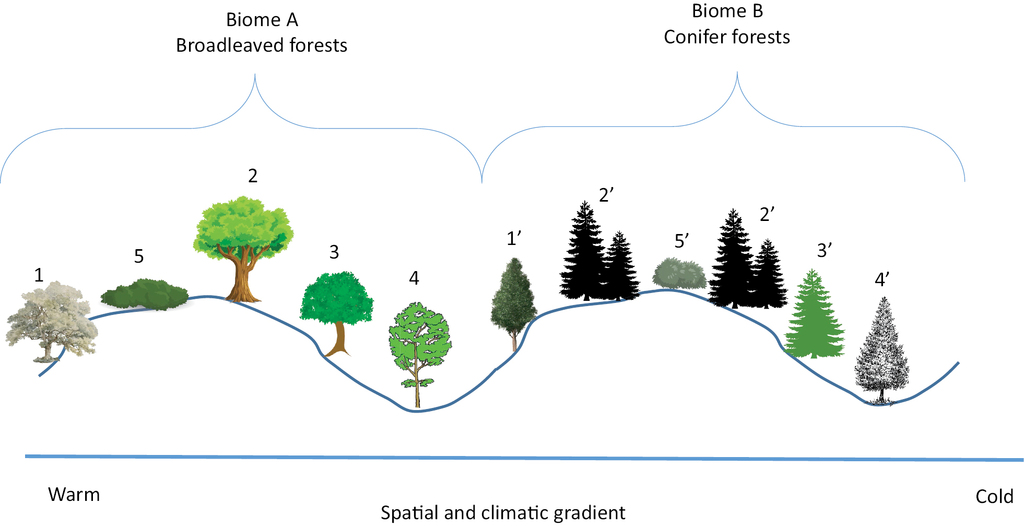
A biome is replaced by a neighboring one when the climate undergoes a substantial change. Sigmeta representative of neighboring biomes A and B. 1 and 1’. Dry and warm biotope. Xerothermophilous. 2 and 2’. Average biotope. Climatophilous. 3 and 3’. Cool and humid biotope. Ombrophilous. 4 and 4’. Riverine biotope. Edafoxerophilous. 5 and 5’. Seral stage.
- Physiognomy or dominant life-forms; e.g., deciduous forests vs. evergreen forests, steppe vs. desert, etc.
- Regional climate or climatic zone; e.g., Ever rainy tropical vs. seasonally rainy tropical, boreal vs. temperate, summer rainy vs. winter rainy, etc.
- Ecological factors; e.g., Soil fertility, natural disturbance regime, etc.
Subbiome. This is a subunit of the biome that is also characterized by physiognomic and climatic features, but with higher resolution. Subbiomes also occur in several continents, but in a few cases, they have a regional distribution in only one continent due to the particular climatic circumstances prevailing. Such is the case for the Patagonian shrubland (5.c) in South America and the Conifer coastal forests (4.b) in North America. We recognize 20 subbiomes in the world.
Bioclimatic classification
Any climatic classification is an attempt to describe the patterns of spatial variation that underlie the mass of accessible climatic data. Each category or climatic type is defined by limits in thermometric and pluviometric values provided by meteorological stations and has a specific territorial expression; i.e., an area in which the values of the climatic factors are within a set of defining limits. If these climatic types are defined in relation to some characteristic flora, fauna or vegetation element, then we will have a bioclimatic classification; i.e., the climatic envelope is justified by or adjusted to the biological content. Likewise, if the climatic types are defined in relation to agricultural contents (viable crops), we will have an agroclimatic classification, and if they are defined in relation to types of soils, the classification will be edaphoclimatic. There are also climatic classifications that are not intended to fit specific content and are purely numerical. Plants are often considered the climatic indicators par excellence because of their sedentary nature and because they necessarily survive the whole series of weather situations that they experience throughout their entire lives. If so, this makes phytoclimatology synonymous with bioclimatology. This fact leads us to think that if we know the climatic limits of a species in a certain territory, we can extrapolate its climatic profile to other areas which are suitable for the survival of that species (bioindication). The first bioclimatic classifications of the 19th century corresponded to the so-called physiognomic plant formations, in which dominant vegetation types were defined by morphological features in large areas of the Earth. Over time, these classifications have been refined and multiplied, and among them we can mention some that have had a greater relevance. An important bioclimatic classification was developed by the German-born Russian meteorologist W. P. Köppen during the first half of the 20th century, which found a notable acceptance (
The types defined in an integrative way by a small number of climatic parameters lead to inflexible systems, in which either the climatic envelope encompasses an often heterogeneous vegetation content, or vegetation entities do not have a corresponding climatic type. For this reason, multidimensional bioclimatic classifications are more practical and more realistic, in which climatic parameters are considered separately, just like the vectors of a vector system.
In the world there are three main geographic belts or zones in each hemisphere: Polar, Temperate and Tropical, separated by the two Polar Circles (66°43'36"N and S) and the two Tropics (23°26'14"N and S). Between Temperate and Tropical, a fourth Aridity Zone is inserted which extends to the center of the continents from their western side.
Adopted general bioclimatic classification of the world
Our proposed classification is inspired directly from that of Rivas-Martínez (
Seasonality, ombroytypes and thermotypes occurring in the different bioclimatic types with approximate threshold values of some climatic parameters. Tp: positive temperature; Pp: positive precipitation (see also Table
| Bioclimatic types | Seasonality | Ombrotypes Io = Pp/Tp | Thermotypes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrobioclimates | Bioclimates | Precipitations: difference between dry and wet season | Temperatures: difference between cold and warm season (Continentality) | Hyperarid < 0,4 | Arid 0,4–1 | Subarid 1–2 | Dry 2–3,6 | Subhumid 3,6–6 | Humid 6–12 | Hyperhumid > 12 | Infra | Termo | Meso | Supra | Oro | Crioro | Icy |
| Warm Tp >2000 | Pluvial BIO15 < 60 | low | null | + | + | + | + | + | + | + | + | + | |||||
| Pluviseasonal BIO15 > 60 | high | low | + | + | + | + | + | + | + | + | + | + | + | + | |||
| Arid Io < 1 | Warm-Temperate nfd < 40 | low | low – high | + | + | + | + | + | + | + | + | ||||||
| Cold nfd > 40 | low | high | + | + | + | ||||||||||||
| Mesic Tp 1000 – 2000 | Aridiestival (Mediterranean s.l.) dws < 1 | high | low – high | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Pluviestival dws > 1; BIO7 < 300 | low | low – high | + | + | + | + | + | + | + | + | + | + | + | ||||
| Steppic BIO7 > 300 | low | high – very high | + | + | + | + | + | + | |||||||||
| Cold Tp <1000 | Tundral-Boreal | low | low – very high | + | + | + | + | + | + | + | + | ||||||
Correspondence between biomes and bioclimatic types (for names of subbiomes, see Table
| Domains and biomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryocratic | 1. Tundra | 1a + 1b + 1c | ||||||||||
| 2. Boreal forest | 2a + 2b | |||||||||||
| Mesocratic | 3. Temperate deciduous forests | 3a | ||||||||||
| 4. Pluvial evergreen forest and shrublands | 4a+4b+4c | |||||||||||
| 5. Scleropyllous-microphyllous evergreen forests and shrublands | 5a + 5b + 5c | |||||||||||
| 6. Steppe | 6a + 6b | |||||||||||
| Xerocratic | 7. Deserts and semi-deserts of arid regions | 7a | ||||||||||
| 7b | ||||||||||||
| 7c | ||||||||||||
| Themocratic | 8. Tropical pluviseasonal foretst and shrublands | 8a + 8b | ||||||||||
| 9. Tropical rain forests | 9a | |||||||||||
| Pluvial | Pluviseasonal | Warm | Temperate | Cold | Steppic | Aridiestival | Pluviestival | Boreal-Tundral | Bioclimates | |||
| Warm | Arid | Mesic | Cold | Macrobioclimates | ||||||||
The thermometric values specify the thermal regime of the locality, as a result of its latitudinal and altitudinal position and the intensity of the solar radiation received. Rain gauge measurements provide the estimates of water availability, basically in the form of liquid water, although in many areas these precipitations are in solid form (snow, hail). Water inputs due to fog condensation, which is substantial in some mountainous areas, are often hidden in the pluviometric data (cryptoprecipitations). Seasonality affects the regular oscillation in the increase and decrease of one or the other according to the seasons of the year. Therefore, there will be a seasonality of rainfall that will be determined by the latitudinal displacement of the aridity and rainfall belts throughout the year, and another of the temperatures, which will be accentuated by the latitude and by the distance from the influence of the seas and oceans.
The system is organized hierarchically, using the factors that exert the greatest influence on the distribution of terrestrial ecosystems. Thus (and adopting the Köppen criterion), a first division is made into four large types or macrobioclimates, reproducing the great climatic belts of the planet that determine the main groupings of ecosystems in the world. From the poles to the equator we distinguish: I. The cold macrobioclimate, II. The temperate macrobioclimate, III. The arid macrobioclimate and IV. The warm macrobioclimate.
I. Cold Macrobioclimate (Cryocratic)
This type is dominated by the low temperatures which fall below zero for at least 4 to 5 months, causing severe limitations to the development of life and enforcing strong adaptation of living organisms to low temperatures. There are two bioclimates within this macrobioclimate. I.a. Tundral. The highest latitudes, oscillating between 55° and 70°, are covered by tundral vegetation in which the trees do not develop due to the short duration of the period of plant activity in the short summers and due to the existence of permanent ice on the ground (permafrost). Permanent ice occupies large areas, especially in Antarctica and Greenland. Continental and oceanic versions can be distinguished. I.b. Boreal. This type occupies the latitudes above a border that oscillates between 40° and 66° depending on the marine currents, where winters are long and cold, and summers are short and rainy. Depending on the amplitude of the seasonal variation of temperatures, oceanic and continental versions can be distinguished. In general, the territory is dominated by cold-resistant coniferous forests, which are accompanied by some deciduous trees, such as birch, willow and aspen. In both cases, there are high elevation versions in the mountains of the temperate and tropical zones.
II. Mesic Macrobioclimate (Mesocratic)
This is the most climatically diverse and the one that hosts the greatest diversity of terrestrial ecosystems. Its lower latitude limit oscillates between 25° and 30°, bordering the warm and arid bioclimates. Its entire area is subject to a thermal seasonality, with a clearly differentiated winter and summer, the more pronounced the greater the latitude and the distance from the influence of the seas. In addition to this, the seasonality of rainfall, basically determined by the polar front, plays an important role in differentiating the bioclimates: II.a. Temperate ombroestival (summer-rainy), occurs in latitudes immediately higher than the tropics and in contact with them. These areas are influenced by the polar front dragged by the westerlies, trade winds and even the monsoonal regime, ensuring summer rainfall in sufficient quantity. There are very oceanic areas, in the regions close to the coast, and others more continental, in which winter rainfall is lower than in summer due to the influence of high polar pressures and, in East Asia, due to the effect of the winter monsoon. It spans Europe, eastern North America, and eastern Asia in the Northern Hemisphere (hereafter NH), while in the south it is found in southern Chile, southeastern sides of Australia and Africa, and New Zealand. The dominant vegetation is temperate deciduous forests and non-sclerophyllous evergreen temperate forests. In the tropical zone, mountains above 1000 to 1500 m asl have a cooler climate and higher precipitations, and thus their climatic conditions approach rainy temperate. II.b. Temperate aridiestival (summer-dry or Mediterranean s.l.), located in the latitudinally lower fringe of this zone (subtropical), between 25° and 45°, on the western sides of the continents and in contact with the extratropical deserts. In the summer there is an intense drought of at least two consecutive months caused by the strengthening and expansion of the subtropical highs, while the winters are under the influence of the polar front that moves towards lower latitudes. The countries bordering the Mediterranean Sea and Middle East, the central-southern area of California, central Chile and Argentinian Patagonia, the Cape region and the south and southwest of Australia correspond to this variant, where the typical plant response is the evergreen sclerophyll-microphyll, both wooded and shrubby. There is a more continental variant of this type extended across central western North America and West-Central Asia. II.c. Temperate steppic, spread over the interior regions of the great continental masses, subject to strong thermal seasonality and low rainfall throughout the year. The powerful winter anticyclones that form on these continents limit the rains, which are somewhat more abundant in summer when there are sporadic incursions of maritime air masses. This climatic variant extends mainly through the interior regions, strongly continentalized, of the great land masses of the Northern Hemisphere (NH), such as that spanning from eastern Central Europe to western China and Mongolia, as well as the interior regions of North America (Great Plains). Its characteristic vegetation is the steppe, formations dominated by grasses, either in mosaic with scattered forest patches (forest-steppe) or without them (grass-steppe).
III. Arid Macrobioclimate (Xerocratic)
This is characterized by climatic aridity and appears in regions with very low rainfall (Ombrothermic Index Io (
IV. Warm Tropical Macrobioclimate (Thermocratic)
This roughly encompasses the regions between the Tropics of Cancer and Capricorn (intertropical), with some subtropical extensions in certain lowlands influenced by warm ocean currents. Within the intertropical zone, all its points receive solar rays with an inclination of 90° twice a year. Therefore, it is the area that receives the most solar energy and is the warmest on the planet, not being subjected to thermal seasonality, although it is to the rainfall seasonality in many of its parts. As for its bioclimates, we distinguish two. IV.a. Tropical pluviseasonal, in which there is a clear seasonality in the rains, which fall mainly in the summer months, leaving a dry season for the remainder of the year, in which the temperatures often reach very high values. It occupies the bands between 5° to 10° and 20° to 25°N and S, to which ITCZ moves on the spring equinox to cause seasonal rains. From the autumn equinox, the movement is towards the opposite hemisphere and the strip comes under the dominion of the high subtropical pressures, giving rise to the dry season. The monsoon regime is also the cause of this climatic variant in the regions where it operates and the trade winds spread this climatic type along the eastern sides of the continents. Vast tropical territories of the Americas, Africa, Asia and Australia are under this variant, where deciduous or evergreen-sclerophyll formations predominate, adapted to survive a hot and arid season. IV.b. Tropical pluvial, in which the rains are abundant for the whole year (aseasonal). It occupies the latitudes closest to the equator, between 10°N and S and is under the permanent influence of the ITCZ, registering high amounts of precipitation and notable thermal uniformity throughout the year, with little or moderate seasonal variation. Its characteristic biome is the tropical rainforest and the typical regions are the Amazon, the Congo basin and the Indo-Pacific archipelagos.
Ombrotypes and thermotypes
Another set of categories, which we call thermotypes and ombrotypes, can be superimposed on this primary classification, in response to the variability caused by the topographic relief within each macrobioclimate and bioclimate described above, particularly accentuated in the case of the mountains. To account for this variability, a series of thermal and ombric types are distinguished separately, following the criterion that was initiated by the French geobotanical school for North Africa (
Values of Io (ombrothermic index, quotient between positive precipitation Pp and positive temperature Tp) for the ombrotypes.
| a) Ombrotypes of the World | |
|---|---|
| Ombrotype | Io = Pp/Tp |
| hyper-arid | < 0.4 |
| arid | 0.4–1 |
| sub-arid | 1–2 |
| dry | 2–3.6 |
| sub-humid | 3.6–6 |
| humid | 6–12 |
| hyper-humid | > 12 |
Values of Pt (positive temperature) for the thermotypes at different latitudes.
| b) Thermotypes of the world | ||||||
|---|---|---|---|---|---|---|
| Latitude | Infra | Thermo | Meso | Supra | Oro | Cryoro |
| 00–10° | >2900 | 2900–2300 | 2300–1700 | 1700–950 | 950–450 | 450–100 |
| 10–20° | >2900 | 2900–2300 | 2300–1700 | 1700–950 | 950–450 | 450–100 |
| 20–30° | >2400 | 2400–2100 | 2100–1500 | 1500–900 | 900–450 | 450–100 |
| 30–40° | >2400 | 2400–2100 | 2100–1500 | 1500–900 | 900–450 | 450–100 |
| 40–50° | 2350–2000 | 2000–1400 | 1400–800 | 800–380 | 380–100 | |
| 50–60° | 1400–800 | 800–380 | 380–100 | |||
| 60–70° | 800–380 | 380–100 | ||||
| 70–80° | 280–100 | |||||
| 80–90° | ||||||
| Bioclimatic variables and indexes |
|---|
| From CHELSA: |
| BIO1 = Annual Mean Temperature (T) |
| BIO2 = Mean Diurnal Range (Mean of monthly (max temp–min temp)) |
| BIO3 = Isothermality (BIO2/BIO7) (×100) |
| BIO5 = Max Temperature of Warmest Month |
| BIO6 = Min Temperature of Coldest Month |
| BIO7 = Temperature Annual Range (BIO5–BIO6) |
| BIO8 = Mean Temperature of Wettest Quarter |
| BIO9 = Mean Temperature of Driest Quarter |
| BIO10 = Mean Temperature of Warmest Quarter |
| BIO11 = Mean Temperature of Coldest Quarter |
| BIO12 = Annual Precipitation (P) |
| BIO15 = Precipitation Seasonality (Coefficient of Variation) |
| BIO16 = Precipitation of Wettest Quarter |
| BIO17 = Precipitation of Driest Quarter |
| BIO18 = Precipitation of Warmest Quarter |
| BIO19 = Precipitation of Coldest Quarter |
| Additional ones: |
| nfd = number of frost days |
| dws = drought of the warm season (BIO18/BIO10) |
| Tp = positive temperature: sum of the mean temperatures of the months in which t > 0, multiplied by 10; ?12i=1 ti when ti > 0 |
| Pp = positive precipitation: sum of the mean precipitations of the months in which t > 0; ?12i=1 ti when ti > 0 |
| Io = Pp/Tp Ombrothermic index by Rivas-Martínez: quotient between the positive precipitation and positive temperature. |
| CI = Coldness Index (Kira) = - ? (t – 5) in the months in which t < 5 |
The comparison of the vegetation belts and climates of mountains at different latitudes has been the focus of attention since the dawn of geobotany (see
Thermotypes altitudinal ranges across latitude. A Theoretical representation; B Real representation of the 610 selected locations classified after thermotypes plotted against altitudes. In the latter, an anomaly is shown in the Infra curve which reaches higher elevations than Thermo and Meso in latitudes between 32 and 36°, meaning that there are locations which are Infra at higher elevations than others which have Tp of Thermo. This can be explained by the deserts occurring in these latitudes, which are subjected to extremely hot summers and enhancing high Tp values.
The occurrence of the thermotypes and ombrotypes in the main geographic zones is shown in Table
Thermotypes and ombrotypes present in the four broad geographic zones of the earth.
| Thermotypes | Tropical | Arid | Temperate | Polar | |||||||
| Pluvial | Pluviseasonal | Warm | Temperate | Cold | Aridiestival | Pluviestival | Steppic | Boreal | Tundral | ||
| Cryoro | |||||||||||
| Oro | |||||||||||
| Supra | |||||||||||
| Meso | |||||||||||
| Termo | |||||||||||
| Infra | |||||||||||
| Ombrotypes | Io | Tropical | Arid | Temperate | Polar | ||||||
| Pluvial | Pluviseasonal | Warm | Temperate | Cold | Aridiestival | Pluviestival | Steppic | Boreal | Tundral | ||
| Hyperarid | < 0.4 | ||||||||||
| Arid | 0.4–1 | ||||||||||
| Subarid | 1–2 | ||||||||||
| Dry | 2–3.6 | ||||||||||
| Subhumid | 3.6–6 | ||||||||||
| Humid | 6–12 | ||||||||||
| Hyperhumid | > 12 | ||||||||||
Effect of the continentality in the thermotypes altitudinal zonation
As long ago established by
Continentality and the elevation of the upper limits of the thermotypes. It is noteworthy that the upper limit of the cryoro is higher at latitudes of 20–30° in the NH and 10–20° in the SH, than in the interval between 20°N and 10°S (Figure
The change of the thermal definition of the thermotypes. The thermotypes are basically defined by the Tp (positive temperature, Table
Tp limits of thermotypes across latitude. As a general pattern, thermotypes shift towards colder limits at higher latitudes, as shown mostly in the Infra, Thermo, Meso and Supra thermotypes. This means that they occupy relatively higher elevations in low latitudes. They also vanish at certain latitudes: above 30° the Infra starts vanishing, above 40° the Thermo, above 50° the Meso, above 60° the Supra, above 70° the Oro and the Cryoro can surpass that latitude, always considering low elevations.
This is a model by
In short, the proposed system provides a typology in which the macrobioclimates reflect the general conditions of the great climatic zones of the earth, the bioclimates the seasonal regimes, the thermotypes the temperature regime (which will depend on the latitude and the elevation of the land above sea level), and the ombrotypes the abundance of rainfall and water availability, which are the climatic patterns determined by the General Circulation Model and the elevation of the terrain.
The climatic parameters and indices used are indicated in Table
Material and methods
The bioclimatic typology is inspired by that of Rivas-Martínez (
The climatic data were extracted from Chelsa Climate database (
Distribution of the 616 localities from which climatic data have been obtained by means of CHELSA. The colors of the dots correspond to the 20 subbiomes and the lines are subbiome boundaries: 1a Polar tundra, 1b Tundras of the temparate mountains in cryoro belt, 1c Tundras of the tropical mountains in cryoro belt, 2a Lowland boreal Taiga, 2b Forests and shrublands of the temperate oro belt, 3a Temperate deciduous forests, 4a Lauroid evergreen forest of the lowlands, 4b Conifer coastal forests, 4c Tropical montane cloud lauroid and conifer evergreen forest, 5a Oceanic sclerophyllous-microphyllous evergreen forests and shrublands (Mediterranean), 5b Continental scrub and woodlands, 5c Patagonian shrubland, 6a Forest-steppe, 6b Grass-steppe, 7a Cold deserts and semi-deserts, 7b Temperate deserts and semi-deserts, 7c Warm deserts and semi-deserts, 8a Tropical xeric shrublands and woodlands, 8b Tropical pluviseasonal forests and woodlands, 9a Tropical rain forests.
The data of the 616 locations are shown in the table in Suppl. material
Description of the world biomes
General patterns of distribution
A summary of the adopted biomes typology is shown in Table
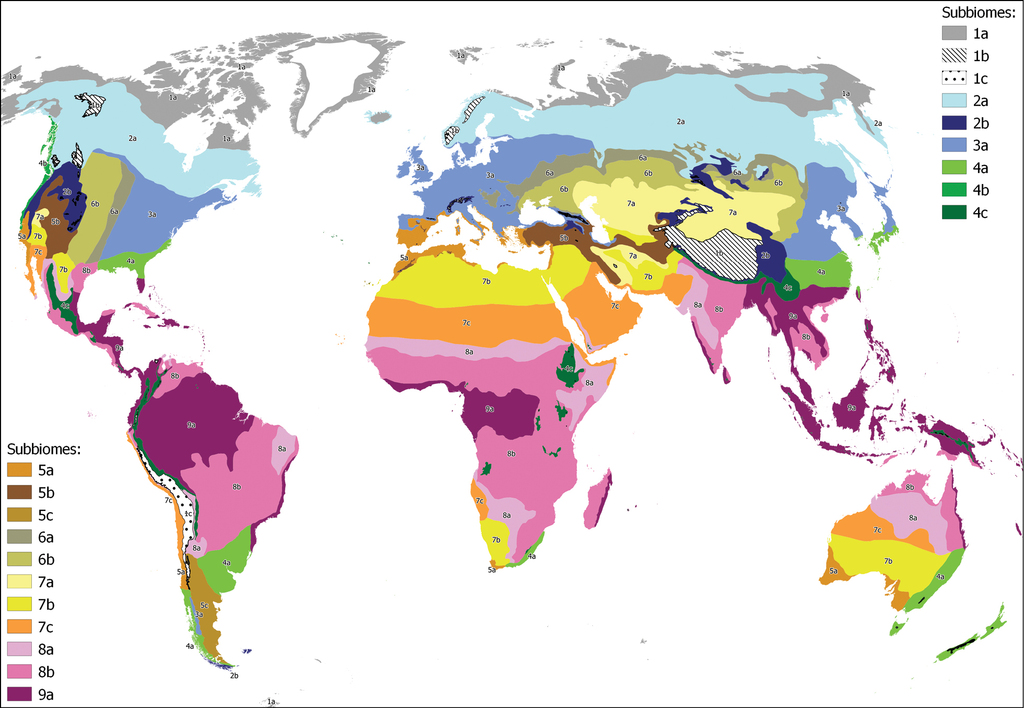
Distribution of the 20 subbiomes across the world. 1a Polar tundra, 1b Tundras of the temparate mountains in cryoro belt, 1c Tundras of the tropical mountains in cryoro belt, 2a Lowland boreal Taiga, 2b Forests and shrublands of the temperate oro belt, 3a Temperate deciduous forests, 4a Lauroid evergreen forest of the lowlands, 4b Conifer coastal forests, 4c Tropical montane cloud lauroid and conifer evergreen forest, 5a Oceanic sclerophyllous-microphyllous evergreen forests and shrublands (Mediterranean), 5b Continental scrub and woodlands, 5c Patagonian shrubland, 6a Forest-steppe, 6b Grass-steppe, 7a Cold deserts and semi-deserts, 7b Temperate deserts and semi-deserts, 7c Warm deserts and semi-deserts, 8a Tropical xeric shrublands and woodlands, 8b Tropical pluviseasonal forests and woodlands, 9a Tropical rain forests.
Representation of real and virtual areas of the 9 biomes across latitude and altitude in profiles of the continents. A and B are North South profiles from the western side of the continents (A The Americas from the Pacific; B Europe and Africa from the Atlantic), and C and D are profiles from the eastern side (C The the Americas from the Atlantic; D Asia and Australasia from the Pacific). E Represents a transect in the middle of the Asian continent, continued with the profile of Africa from the Indian Ocean. Inland the colors are darker than in the air, where they only represent a virtual area. 1 Tundra; 2 Boreal forest; 3 Temperate deciduous forest; 4 Temperate pluvial evergreen forest, shrublands and grasslands; 5 Temperate aridiestival evergreen forests and shrublands; 6 Steppes; 7 Deserts and semi-deserts of arid regions; 8 Tropical pluviseasonal forests and shrublands; 9 Tropical rain forests.
| Domains | Ecozones | Biomes | Subbiomes |
|---|---|---|---|
| A. Cryocratic Domain of the cold climates | AA. Polar and boreal ecozone | 1. Biome of the tundra | 1a. Polar tundra |
| 1b. Tundras of the temparate mountains in cryoro belt | |||
| 1c. Tundras of the tropical mountains in cryoro belt | |||
| 2. Biome of the boreal forest | 2a. Lowland boreal Taiga | ||
| 2b. Forests and shrublands of the temperate oro belt | |||
| B. Mesocratic Domain of the temperate climates (incl. tropical mountains) | BA. Temperate ombroestival ecozone | 3. Biome of the temperate deciduous forests | 3a. Temperate deciduous forests |
| 4. Biome of the temperate pluvial evergreen forest, shrublands and grasslands | 4a. Lauroid evergreen forest of the lowlands | ||
| 4b. Conifer coastal forests | |||
| 4c.Tropical montane cloud lauroid and conifer evergreen forest | |||
| BB. Temperate aridiestival ecozone | 5. Biome of the temperate aridiestival evergreen forests and shrublands | 5a. Oceanic sclerophyllous-microphyllous evergreen forests and shrublands (Mediterranean) | |
| 5b. Continental scrub and woodlands | |||
| 5c. Patagonian shrubland | |||
| BC. Temperate hypercontinental steppic ecozone | 6. Biome of the steppe | 6a. Forest-steppe | |
| 6b. Grass-steppe | |||
| C. Xerocratic Domain of the arid climates | CA. Ecozone of the deserts and semi-deserts of arid regions | 7. Biome of the deserts and semi-deserts of arid regions | 7a. Cold deserts and semi-deserts |
| 7b. Temperate deserts and semi-deserts | |||
| 7c. Warm deserts and semi-deserts | |||
| D. Thermocratic Domain of the warm climates | DA. Tropical pluviseasonal, rainy and dry seasons | 8. Biome of the tropical pluviseasonal forests and shrublands | 8a. Tropical xeric shrublands and woodlands |
| 8b. Tropical pluviseasonal forests and woodlands | |||
| DB. Tropical pluvial, rainy all the year round ecozone | 9. Biome of the tropical rain forests | 9a. Tropical rain forests |
| Macrobioclimates | Cold | Mesic | Arid | Warm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermotypes | Cryoro | Oro | Cryoro | Oro | Supra-Meso | Termo-Infra | Cryoro | Oro | Supra-Infra | Cryoro | Oro | Supra | Meso-Infra |
| Subbiomes | |||||||||||||
| 1a. Polar tundra | X | ||||||||||||
| 1b. Tundras of the temparate mountains in cryoro belt | X | ||||||||||||
| 1c. Tundras of the tropical mountains in cryoro belt | X | ||||||||||||
| 2a. Lowland boreal Taiga | X | ||||||||||||
| 2b. Forests and shrublands of the temperate oro belt | X | ||||||||||||
| 3a. Temperate deciduous forests | X | X | |||||||||||
| 4a. Lauroid evergreen forest of the lowlands | X | ||||||||||||
| 4b. Conifer coastal forests | X | X | X | ||||||||||
| 4c. Tropical montane cloud lauroid and conifer evergreen forest | X | X | |||||||||||
| 5a. Oceanic sclerophyllous-microphyllous evergreen forests and shrublands (Mediterranean) | X | X | |||||||||||
| 5b. Continental scrub and woodlands | X | X | |||||||||||
| 5c. Patagonian shrubland | X | ||||||||||||
| 6a. Forest-steppe | X | ||||||||||||
| 6b. Grass-steppe | X | ||||||||||||
| 7a. Cold deserts and semi-deserts | X | X | |||||||||||
| 7b. Temperate deserts and semi-deserts | X | ||||||||||||
| 7c. Warm deserts and semi-deserts | X | X | |||||||||||
| 8a. Tropical xeric shrublands and woodlands | X | ||||||||||||
| 8b. Tropical pluviseasonal forests and woodlands | X | ||||||||||||
| 9a. Tropical rain forests | X | ||||||||||||
A. Cryocratic Domain of the cold climates
The climate of this domain is governed by the clearly negative radiative energy balance, with more radiation issued than received. This entails the permanence of the polar high pressures, which diffuse extremely cold air masses causing low temperatures and long frost periods in the higher latitudes of the Planet, mostly above 60°N and S. The long cold periods alternate with brief and cool summer seasons which can be longer and warmer in the case of the boreal forest. As a general rule, the values of Tp units rarely exceed 1000. In the case of the tundra, summers are practically non-existent since the temperature does not exceed 10°C on average in the warmest month, being also short but mild in the case of the taiga. The differences between winter and summer are extreme, with very long daylight in the central days of summer, which lasts up to 24 hours on the corresponding days in the latitudes above those of the Arctic and Antarctic Polar Circles, while in winter the situation is the inverse. There is a great difference between the seasons regarding the solar radiation received.
The vascular vegetation of this domain presents the entire syndrome of adaptations to low temperatures. Its origin is quite recent (
AA. Polar and Boreal ecozone
This is the only ecozone of the domain and it can be split into the tundra and the boreal forest biomes. They both present clear relationships with the high elevations of the temperate and tropical mountains, which are also under the dominance of the low temperatures and are considered a part of this ecozone and its corresponding biomes.
1. Biome of the tundra.
The term derives from the Russian тундра, which is applied to a plain area without trees, because it is a biome devoid of trees and constituted by a continuous, dense and evergreen vegetation layer formed by a combination of bushes, herbaceous vascular plants (often grasses and sedges), bryophytes and lichens; the low temperatures prevent the development of trees. It is the biome with the greatest adaptation to cold climate, both in latitude and altitude. Temperatures remain above freezing for only 2 to 6 months, during which the probability of frost does not disappear. The average temperature of the warmest month does not generally exceed 10°C. Ocean influences determine the span of temperature seasonality, being much higher in continental regions. In addition to the Arctic and Antarctic tundra, the cryoro (alpine) belts of the world’s mountains above the forest level are considered to have tundral vegetation due to similarly low temperatures and the morphologic analogies with them. The upper limit of the polar tundra is marked at 380 units of positive temperature (Tp) and 450 in the case of tropical and Mediterranean alpine tundras.
1.a. Polar (Arctic and Antarctic) tundra.
This extends through the territories closest to the poles above a border that oscillates between 65° and 70° (exceptionally it can go down to 56°) of latitude, depending on the continentality and the marine currents, following more or less the isotherm of 10°C of the month of July (January in the SH). It spans vast peri-arctic territories in the northernmost fringes of North America (Alaska, Canada, and Greenland) and Eurasia. In the SH there are few emerged lands in those latitudes but it is recognized in the extreme north of the Antarctic peninsula and in the small islands that surround that continent.
Due to the low temperatures and its recent release from the ice cover since the last glacial maximum, the soils of the tundra are poorly developed. In polar tundras the characteristic soil is permafrost, which freezes completely in the long winter and thaws only on its surface (mollisol) in the short summer, always leaving a permanently frozen layer of soil below 25 to 30 cm, which can reach a remarkable depth (pergelisol). Permafrost is one of the elements that limit the growth of trees by preventing the development of their root system. In the domain of the tundra there are the so-called polygonal soils. These are formations of geometric cellular figures of different sizes, with circular to hexagonal contours, formed by the accumulation of larger particles displaced by the freeze-thaw cycle.
In Arctic tundras rainfall is low, of the order of 200 mm per year or even less, and is mostly in the form of snow, so that a layer of snow forms lasting all winter. With the spring thaw this ice melts and in the short summer there is abundant availability of liquid water. The wind is responsible for an uneven distribution of the snow on the surface, so that it accumulates in the hollows and is swept from the ridges. This is of great importance in the distribution of the mosaic of tundral communities, as the most protected areas will be in the troughs and the most exposed at elevation.
Because of the extreme specialization required to survive in such an extreme habitat, tundras are generally species poor. The Arctic tundras support approximately 900 species while the cryoro (alpine) tundras are more diverse as they have numerous endemics typical of the mountains. Woody species of the genera Arctostaphylos, Betula (B. nana), Cassiope, Dryas, Empetrum, Ledum, Salix, Silene acaulis, Vaccinium etc., herbs of the genera Carex, Deschampsia, Saxifraga (S. oppositifolia) or Silene are frequent in the Arctic tundras. Non vascular plants are abundant such as the bryophytes Hylocomnium splendens and Polytrichum juniperinum or fruit lichens such as Cetraria nivalis, Cladonia gacilis or Cladina mitis (
In Antarctica, tundral vegetation has a modest representation in the Antarctic Peninsula area with only two vascular species: Colobanthus quitensis and Deschampsia antarctica (
1.b. Tundras of the temperate cryoro (alpine) belt.
In the tundras of the cryoro (alpine) belt of the mesic (temperate) zone, the thermic and luminic seasonality is lower than in the polar regions, reaching to nill in the tropical latitudes. In contrast, there is a strong daily temparature oscillation (diurnality) and a strong incidence of wind, with its intense, abrasive and drying effect upon the areas not sheltered by the snow cover. As a result, the cryoro tundras have a less severe winter and in general receive higher rainfall due to the orographic precipitations effect. Permafrost is thinner in the cryoro tundras, dissapearing in the mid latitudes where permanently iced soils are lacking. In the cryoro belt tundras, periglacial phenomena associated with movements caused by the daily cycle of melting-freezing of soil water, such as cryoturbation and solifluction, are frequent, as well as the fragmentation of rocks due to freezing of water: gelifraction (
In the alpine (cryoro) tundras, rainfall can be very variable oscillating between low and very high values, depending on the climatic regime of the mountain system in question and the accumulation of snow as redistributed according to relief by the strong winds.
As the cryoro tundras are at higher elevations as latitude decreases, uv radiation increases, favouring the differentiation of new species and endemics. Thus, cryoro tundras have a particular flora resulting from the evolution of the low elevation local floras drawn by the adaptation to high mountain environments created during mountain uplift. This is particularly relevant in the mid and lower latitudes, such as the Mediterranean climatic area. In the higher latitude mountains, there is a strong participation of polar tundra flora which merge with the local mountain flora, due to migrations that took place in the Pleistocene ice ages, when the alpine tundras contacted with the polar tundra in the mid elevation mountains of the high latitudes, allowing floristic exchange between both elements (
In the SH there are fewer mountains bearing cryoro tundras in temperate latitudes. We can mention the Southern Andes, above the tree line, where there is vegetation of dwarf scrubs and herbs, with species such as Gaultheria mucronata, Gunnera magellanica, Hamadryas magellanica, Marsippospermum grandiflorum, Oreopolus glacilis, etc. (
1.c. Oro-Cryoro Tundras of the tropical mountains.
The tropical mountains show a high uniqueness because their floristic lineage is different from that of the cryoro tundras of the extratropical mountains (
2. Biome of the boreal and austral forest
This biome is often known by the name of taiga, from Russian тайгá, being a biome dominated by conifer forests, mostly perennial but which also include some deciduous broadleaved trees such as birches, aspen and willows. It is subjected to a shorter freezing period than the tundra, having a frost-free season of a certain duration which allows the soils to thaw at least partially and permit trees to establish. Temperatures reach very low values during winter, often lower than in the tundra, due to the high continentality of some of the areas occupied by this biome (
The typical zonal soil of the taiga is the podzol, which is created by the acid organic matter (mor) produced by the conifers and ericoids which grow under these climatic conditions and on acidic siliceous bedrock. On other substrates, such as volcanic rocks, andosolic podzols will be found.
2.a. Lowland boreal taiga.
The boreal taiga forest is predominantly a conifer forest, with some broadleaved trees, ericoid shrubs and an herb layer with sedges and grasses as well as bryophytes and lichens. Mycorrization is widespread due to the soil poverty. The boreal taiga develops in areas of higher thermic conditions and in lower latitudes than the Arctic tundra. It expands uninterruptedly across the NH lands both in Eurasia (Fennoscandia and northern Russia including large areas in Siberia) and North America, mostly in Alaska and Canada, in latudes oscillating between 42° and 72°N. The climate is cold with a high temperature seasonality having long and cold winters in which temperatures reach extreme negative values, particularly in some areas as Eastern Siberia (Yakutia); there are at least 120 days where temperatures are < 10°C. On the other hand, the short summers can have 30 to 120 days in which temperatures are above 10°C, and with some days of remarkable length (
In north Europe the most frequent boreal forests conifers are Larix decidua, Picea abies and Pinus sylvestris, being accompanied by the broadleaved Betula pubescens and Populus tremula. In east Siberia Abies sibirica, Larix gmelinii, L. sibirica, Picea obovata, Pinus pumila or P. sibirica are found, often with the broadleaved trees Betula ermanii, B. dahurica and B. platyphylla. South of the taiga, in some areas of Europe and East Asia conifers mix with deciduous temperate tree species resulting in a transtional forest zone called hemiboreal (
The boreal forest of northwestern America is formed by conifers such as Larix laricina, Picea glauca, P. mariana and Pinus banksiana, while in the northeast Abies balsamea, Betula occidentalis, B. papyrifera, Populus balsamifera and P. tremuliodes form the broadleaved element.
The understorey flora is formed by species of the genera Arctostaphylos, Empetrum, Ledum, Rhododendron, Vaccinium, Pyrola, Linnaea, Lycopodium, Trientalis and several orchids such as Corallorhiza trifida. Bryophytes and lichens are abundant (Weber and van Cleve 2005).
2.b. Forests and shrublands of the temperate zone oro (subalpine) belt.
In the oro (subalpine) belt of the mountains of the temperate geographic zone, below the cryoro (alpine) level, this biome can be recognized as a mountain variant or subbiome dominated by conifers (
For the conifer forests of the European mountains, we can mention Larix europea, Picea abies, Pinus cembra, P. mugo and P. uncinata for the temperate pluviestival regions, while in Mediterranean latitudes Pinus nigra subsp. salzmanii and P. sylvestris (several varieties), as well as Cupressus sempervirens, Juniperus hemisphaerica and J. sabina, are typical (
In the mountains of the temperate aridiestival areas (Mediterranean), oro (subalpine) levels are often occupied by a thorny cushion shaped shrubland in which several spiny legume species of Astragalus, Echinospartum, Erinacea and Genista dominate. In some territories, this shrubland is dotted with scattered populations of pines, cypresses or junipers.
In southern Tierra del Fuego, a small area is subjected to a climate corresponding to that of the Boreal forest biome – in a very oceanic variant – where the vegetation is dominated by broadleaved Nothofagus species (Magellanic forest) combined with dwarf conifers of the genera Araucaria, Austrocedrus, Fitzroya, Pilgerodendron or Podocarpus, in a mosaic with extensive moss-mire areas. This unit has been named the Magellanic Antiboreal Evergreen Forest (
B. Mesocratic Domain of the temperate climates
The temperate zone of the earth extends between the high latitude zone dominated by cold polar air masses and the intertropical zone where solar radiation is maximum. In it, the thermal seasons are clearly distinguished between a hot summer and a colder winter, which coincide with the contribution of major-minor radiation throughout the year. This seasonality diminishes in the vicinity of the tropics (subtropical areas), particularly in terms of the rigors of winter. Within this temperate zone, the climatic regime is governed by the influences of the Polar Front, the Subtropical Highs and the Trade Winds depending on the regions. All these regimes are subject to seasonal latitudinal displacements, so that in the winters of the NH there is a displacement of the whole set towards the south, and in summers it is the other way around. This temperate zone is subject to an extreme diversity of climatic situations, particularly in the NH, where there is a large extension of landmass compared to the SH. The northern land masses (Eurasia, North America, North Africa) cover a substantial part of the total area and are traversed by many mountain ranges permitting the effect of continentality to play a significant role in the central regions of these continents. We include in this domain the areas that register an Io index above 1 and a positive temperature Tp ranging between 1000 and 2000 units approximately, where the vegetation can develop up to a complete coverage of the ground surface, leaving the areas below that level in the desertic area of the arid climates. In the tropical latitudes, similarly to the cryocratic domain, the mountains replicate the mesocratic conditions above 1500 m of altitude due to the decrease in temperatures (meso-supra levels). For that reason, the ecosystems that inhabit areas above that altitude can be included in this domain since the decrease in temperatures is accompanied by the existence of many extratropical lineages, both of Holarctic and Gondwanan origin. The vegetation consists of a variety of forests, groves, bushes or grasslands, depending on regional conditions.
BA. Temperate pluviestival ecozone
The summer season is critical in this domain because is the period in which plants can grow and reproduce. There is a phenological adjustment to seasonality of the entire ecosystem. Thus, the availability of moisture permits intense development and growth providing favorable conditions for high biomass production. In this category could also be included temperate rain forests as have been mentioned in the literature particularly for the Pacific coast of the Americas (
3. Biome of the temperate deciduous forest.
Rainy summers in combination with a clear thermic seasonality, with well differentiated summer and winter seasons, allow the dominance of deciduous woody plants which shed their leaves in the severely cold season (criodeciduous).
3.a. Temperate deciduous forests.
This represents the only subbiome of this biome. The climatic conditions for this biome-subbiome are well represented in the main three areas of the NH continents: East Asia, East North America and Western Eurasia. In the literature, this biome-subbiome has been also called the nemoral forest (
The eastern North American region is favored by the rainfall linked to the maritime Atlantic air masses brought mostly by the northern Trade Winds. The vast area covered by this biome in North America spans between the Canadian areas of the St. Lawrence river and the Great lakes to the southern Appalachians, and from the east coast to the middle Mississippi basin, between 32° and 45°N latitude and 70° to 98°W longitude (
In temperate East Asia, this biome is widespread form northeastern China to the southern sectors of the Russian Far East, including most of both the Korean peninsula and the Japanese archipelago (
In Western Eurasia this biome covers a large area encompassing most of Europe, a strip in central-western Siberia, the Caucasus piedmonts and the eastern and southern coastal fringe around the Black Sea (Euxinian) and south Caspian Sea (Hyrcanian), a vast area limited by the boreal forest in the north and the Mediterranean and steppic regions in the south (
In the SH there are only a few scarce relictual representations of this biome. This is likely due to the lower continentality of the SH emerged lands in comparison to those of the north (
4. Biome of the temperate pluviestival evergreen forest, shrublands and grasslands.
This biome encompass highly diverse forms of dominant vegetation formed by broadleaved evergreen species as well as conifers and grasslands. It is often formed by perennial evergreen forests, with leaves of varied texture and size, often coriaceous with shiny surfaces, called lauroid (laurisilvae), but also conifers of various taxa. This biome is a complex and diverse one as it is represented in a variety of territories across the temperate and tropical latitudes of the world in both hemispheres.
In the temperate zone, it occupies the space between the tropics and the nemoral deciduous forest areas, under high to mid oceanic conditions entailing moderate winters and high summer precipitations. In the tropics it is also represented at the mid-elevations (meso-supra) of the mountain systems where temperatures are colder than in the lowlands. Due to orographic precipitations and frequent fogs, tropical mountains support this biome under several variants (Afromontane, American Monteverde, etc.). In spite of such a diversity and broad geographic distribution, there are sufficient features to recognize a certain unity in physiognomy and origin between them. There are three subbiomes to be distinguished within it. In some coastal areas of the western side of the continents, particularly in Chile and in northwestern North America (British Coumbia and Alaska), local conditions related to the persistence of westerlies allow the subsistence of this biome in lowlands or low elevation ranges in latitudes up to 55 and 60° respectively. In Western Europe, it may be expected to find such a biome in the comparable latitudes of some coastal areas, but it is not present. This absence may be due to the extinctions that happened during the Pleistocenic ice ages.
4.a. Lauroid subtropical evergreen forest of the lowlands.
This subbiome can be found in a zonal position in the lowlands of the coastal or ocean influenced areas of the extratropical continental land masses. In the eastern side of the continents, the summer low pressure in mid latitudes circulates oceanic air masses towards the continent in a monsoonal regime causing abundant summer rains coincident with high temperatures. Winters are relatively dry and cold as the situation is inverse and land air dominates causing relatively low temperatures and scarce precipitation. Nevertheless, tempertures remain above 5°C in the coldest month. These formations have received several names such as subtropischer Lobeerwald in German, temperate broad-leaved evergreen forests in English (
In the Northern Hemisphere:
Eastern Asia is the most extensive area occupied by this subbiome, between 25° and 35°N latitude. Occupies the major part of Eastern continental China and the island of Formosa (Taiwan), penetrating to the west over the ranges connecting with the Tibetan Plateau and the arid regions of Central Asia, being limited by the 99° and 123°E longitude. Northwards, it reaches the southern areas of the Korean peninsula and the Japanese archipelago, in the coastal districts of the islands of Kyushu, Shikoku and southern Honsu (Yong-Chang and Liang-Jun 2016). In the south, it is constrained by the tropical rain forest (biome 9) in a transitional fringe north of Guangzhou (Canton) and connects to the west with the long narrow zone which occupies the southern humid mid-elevation slopes of the Himalayas (
This region is subjected to a monsoonal regime with warm humid air masses comning in summer from the east (Pacific, Seas of China) and to winter invasions of cold air from the center of the Asian Continent originated by the strong Siberian high, which are responsible for brief cold episodes that can cause snow events.
The forests in this region have been dramatically reduced by human pressure, but do survive in some areas and support a high number of tree species of families such as Fagaceae (Castanopsis, Cyclobalanpsis, Lithocarpus, Quercus), Myrsinaceae (Ardisia), Lauraceae (Cinnamomum, Machilus, Persea), Magnoliaceae (Magnolia, Michelia), Symplocaceae (Symplocos), Theaceae (Camellia) etc. In spite of anthropogenic reduction, these forests ere substantially more diverse in their tree flora compared with the eastern North American and Western Eurasian homologous portion (
Southeastern North America is another of the regions where this subbiome is zonal, occupying a vast area in the USA between SW Texas and North Carolina, encompassing southern Louisiana, Mississippi, Alabama, Georgia, northern Florida and South Carolina. This area is under the influence of the Gulf Stream which flows across the Gulf of Mexico and the Atlantic coast and is associated with summer precipitations brought by the eastern trade winds. The forests of this region are substantially poorer than the homologous forests of East Asia, likely due to the higher intensity of the Pleistocenic extinctions that took place in North America. This relative poverty can be also related to the lower precipitations occurring in this region (
In the Southern Hemisphere:
The South African southeast, also called “Southern African Afrotemperate Forest” (
The region of Southeastern Brazil and the Pampas expands between 23° and 39°S latitude, on the Atlantic side of the South American continent encompassing the adjacent countries of the Río de la Plata estuary including southeastern Brazil (Paraná, Santa Catarina, Rio Grande do Sul), Uruguay and Northeastern Argentina. It is the SH counterpart of SE USA. The southern part of this territory is occupied by the Pampas, from Rio Grande do Sul, Uruguay to NE Argentina. It is a vast area which is currently almost entirely occupied by grasslands and crop fields; the arboreal vegetation is thus restricted to marginal positions. The anthropic origin of these grasslands has been previously discussed, as this area experiences completely different climatic conditions to those of the steppes. The overwhelming dominance of the grasslands indicate that the environmental conditions are optimal for them (
The remnants of forest in these areas are formed by some species of regional distribution such as Acacia bonariensis, Cassia corymbosa, Baccharis articulata, Colletia paradoxa, Ocotea acutifolia, Sambucus australis, Schinus longifolius, Scutia buxifolia and particularly the ombú or Phytolacca dioica and the palm Butia yatay. Other species of broader distribution are Acacia caven, Aloysia gratissima, Baccharis tandilensis, Celtis ehrenbergiana, Jodina rhombifolia, Prosopis alba, Zanthoxylum fagara, Margyricarpus pinnatus etc.
The grasslands are dominated by grasses of the genera Andropogon, Aristida, Briza, Bromus, Eragrostis, Melica, Panicum, Paspalum, Piptochaetium, Poa and Stipa, which share their dominance with other herbaceous and woody plants of other families such as Adesmia, Alicropsis, Aster, Baccharis, Berroa, Chaptalia, Heimia, Margyricarpus, Oxalis, Vicia, etc.
This region is continued with the mid elevation areas of Paraná and Santa Catarina in south Brazil, where Araucaria angustifolia and Podocarpus lambertii occur with Dicksonia sellowiana in the understorey, being included in this subbiome due to the subtropical climatic conditions dominant in these territories (
In Southeastern Australia, this subbiome is represented in the coastal strip of land east of the Great Dividing Range, which crosses the eastern side of the continent and separates the arid regions of the interior from the moist coastal areas that are affected by the humid air masses from the Pacific Ocean. The southern stretch of this strip, which extends from the Rockhampton district to the southeastern tip of the continent and the island of Tasmania, is covered by this unit. The climate is humid all the year round and winters are mild. A high representation of species of the genera Acacia and Eucalyptus is present, some of them of great size such as Eucalyptus globulus, E. obliqua and E. regnans, reaching 100 m in stature. Other taxa are Atherosperma moschatum, Doryphora sassafras, Ceratopetalum apetalum, and conifers such as Athrotaxis laxifolia, Phyllocladus aspleniifolius and the endemic Wollemia nobilis, as well as several species of Callitris, Macrozamia and Podocarpus. Some Antarctic remnants are present such as Nothofagus cunninghamii, or the tree ferns Cyathea australis and Balantium (Dicksonia) antarcticum (
The New Zealand archipelago is located in a position where abundant rainfalls occur all year round, particularly on the western side of the islands. This entails a high oceanity which permits the existence of evergreen temperate forests in the whole archipelago lowlands and midlands. The lower levels are occupied by a conifer/broad-leaved forest: among the conifers we can mention Agathis australis, Dacrycarpus dacrydioides, Dacrydium cupressinum, Libocedrus bidwillii, Phyllocladus trichomanoides, Podocarpus totara, Prumnopytis ferruginea and others, while among the broadleaved there are Laurelia nova-zelandiae, Metrosideros umbellata, Weinmannia racemosa, W. silvicola and the palm Rhopalostylis sapida. Several species of tree-ferns of the genera Cyathea and Dicksonia occur in the understory. The evergreen southern beeches (Nothofagus) occupy higher elevations than the conifer/broad-leaved forest with species such as Nothofagus fusca, N. menziesii, N. truncata or N. solandri (
Southern Chile. In the temperate southern part of Chile, around the 38°S, the climate shifts from aridiestival to pluviestival under the influence of the westerlies of the South Pacific. This region extends to Tierra del Fuego at 54°S, because conditions remain extremely oceanic all along the strip. Only in the southwesternmost strip of the area will occur boreal conditions (called antiboreal by local authors such as
4.b. Conifer coastal forests.
This subbiome is also zonal and differs from the previous one in that it presents a high dominance of conifers although there are always a significant presence of evergreen hardwoods. It can be recognized in northwestern North America, where it is known as the Pacific Northwest Forest (
4.c. Tropical and subtropical montane cloud lauroid and conifer evergreen forest and shrublands.
This subbiome can be recognized in the mid elevations of the tropical and subtropical mountains above 1000–1500 m (meso, and supra, humid and hyperhumid). They are evergreen forests formed by broadleaved trees and conifers which receive high amounts of moisture in the form of rain and fog. Conifers and broadleaved trees are both almost always present but they alternate in dominance, mostly depending on the historic evolution of the area. In any case, the broadleaved trees often have coriaceous and shiny leaves (lauroid) while the conifers often bear larger and softer leaves than in other biomes.
The Macaronesian archipelagos: Azores, Madeira and Canaries. Fragments of this subbiome (monteverde) are found in the north Atlantic volcanic archipelagos (Macaronesia), west to the Eurasian continent, being dispersed across most of its islands. Due to the influence of the Azores High, this area is overall subjected to a climatic dynamism in which summer is the driest season. This can be locally offset by orogaphic precipitations and, above all, through the cryptoprecipitations that take place due to the condensation of the fogs accumulating in the mid elevations of the windward slopes facing the trade winds (sea of clouds). Rain increases northwards and westwards so that the Azores are the most humid and the Canaries the driest, being the monteverde generally in the former and restricted to the north facing slopes in the latter. This monteverde or laurisilva (
Trees are all perennial, with Apollonias barbujana, Clethra arborea, Heberdenia bahamensis, Ilex canariensis, I. perado, Myrica faya, Laurus azorica, L. novocanariensis, Ocotea foetens, Persea indica, Picconia excelsa, Prunus lusitanica and Visnea mocanera being the most relevant among others. Some of the noteworthy ferns are Culcita macrocarpa, Davallia canariensis and Woodwardia radicans.
The southern slopes of the Himalayas are covered by this subbiome at mid-elevations between 1000 m and 2500 m, above the tropical foothills that precedes the Indo-Gangetic plain covered with biomes 8 and 9, and below the subalpine conifer forest of 2.b thriving in the upper altitudes. This territory forms a long and narrow zone that spans along the southern slopes of the Himalayas, receiving the copious rains of the summer monsoon. This strip is thought to be the original area of the tea plant (Camellia sinensis) and is characterized by rhododendron forests, of which there are several tree species. Among them, Rhododendron arboreum, the national flower of Nepal, stands out, but also can be mentioned R. barbatum, R. campanulatum or R. lepidotum (
Afromontane forests occur at elevations above 1000 m asl, where rainfall usually exceeds 1200 mm and fogs are frequent throughout the year. Their distribution is discontinuous being found in the long mountain chain crossing eastern Africa (
In the Indo-Malesian region, In the mountains of Borneo, Java, Sumatra and other islands, the flora is derived from both Asian and Australian lineages of families, such as Araucariaceae, Clethraceae, Ericaceae, Fagaceae, Lauraceae, Myrtaceae, Podocarpaceae, Symplocaceae and Theaceae. They are very rich in epiphytes and forbs in the understory, with genera such as Begonia, Cyrtandra and Musa. Some species of this SW Asian region are Agathis dammara, Dacrycarpus imbricatus, Castanopsis buruana, Lithocarpus celebicus, Magnolia carsonii, Myrsine affinis, Pinus kesya, Prunus mirabilis etc. (
In the mountains of New Guinea, there are up to 14 species of Nothofagus which comprise forests from the mid elevations up to 3000 m (Robbins 1961;
The island of New Caledonia is a fragment of Gondwana which has migrated northwards, entered into the intertropical zone and currently is immersed in a tropical climate. However, the indigenous flora is largely derived from the temperate Gondwanan and retains up to five species of Nothofagus and numerous gymnosperms (
In the Indian subcontinent, these forests are recognized in the mountains of the south of the country, in the chain known as the Western Gaths, between the states of Kerala and Tamil Nadu (
In Meso and South America there are important areas with montane forests where temperatures are lower than in the lowlands, precipitations higher and fogs frequent. In Meso- and Central America there are the Mexican Sierra Madre Oriental and Occidental, as well as the Mountains of Central America. In the Mexican sierras, above 1500 m asl of elevation, precipitations increase and are concentrated in the central months of the year (summer). There thrive evergreen lauroid forests, in particular the locally called Mesophylous Montane Forest (
The Guiana Shield reaches elevations of ca. 3000 m asl in the Guiana Highlands, forming a mountainous area known as the Tepuys, which is covered by a vegetation very rich in endemics. Some frequent species are Catostemma durifolius, Pouteria rigida, Protium neglectum, etc. There are also several species of Bonnetia, such as B. roraimae, B. steyermarkii or B. tepuiensis, living above 2000 m asl (
In the northern Andes, in Ecuador, Colombia and Venezuela, the montane forests have species such as Aegiphila bogotensis, Cecropia telenitida, Quercus humboldtii, Saurauia ursina, Vallea stipularis, Weinmannia tomentosa, and several species of the genus Cinchona, such as C. calisaya, C. macrocarpa, C. officinalis, C. pubescens etc. (
BB. Temperate aridiestival ecozone
Unlike the previous ecozone, if the summer is dry (at least 2 months) the ecosystem is subjected to double stress, that of cold in winter and drought in summer, severely limiting biomass production and inducing adaptations to drought in the traits of plants. This summer aridity is due to the fact that in that season these areas are under the influence of the subtropical highs, since they are in the vicinity of the domain of arid climates and desert biomes, in the zonal scheme, or are in rain shadow during the summer season.
5. Biome of the temperate aridiestival evergreen (Mediterranean) forests and shrublands.
This biome lives under Mediterranean climatic conditions (s.l.), and the vegetation is formed by trees, shrubs and scrub with reduced and hard leaves (Tomaselli 1995). This sclerophylly, combined with microphylly in the leaves and other photosynthetic organs, is an adaptation to survive under the arid conditions of the summer months. There are also an abundance of ephemeral therophytes which present a life cycle adapted to this type of climate.
5.a. Oceanic sclerophyllous-microphyllous evergreen forests and shrublands (Mediterranean s.str.).
The territories which are under oceanic conditions, not distant from the seashores, are those which are considered as genuine Mediterranean. There are five areas in the world which can be included in this subbiome: the Mediterranean coastal countries of Europe, Near East and North Africa, the central and southern Californian area, the central Chilean region, the fynbos in the Cape province of South Africa, and the southwestern Australian territories.
The Mediterranean Basin territories extend along the shores of the Mediteranean Sea encompassing relevant areas in southern Europe, from Portugal to Greece, northern Africa (Magrebian countries and Cyrenaica), the Middle East from Anatolia to Siria and Palestine to the Gaza Strip and all the islands within that sea. The territory is covered by sclerophyllous-microphyllous vegetation (
Central and southwestern California is another region where this subbiome can be recognized, having a clear summer drought that is responsible for sclerophyll vegetations types. These forests are usually formed of oak and pine species such as Quercus agrifolia, Q. chrysolepis, Q. douglasii, Q. dumosa, Q. wislizenii, Pinus sabiniana and others, and the shrublands, locally called chaparral, have a high number of species of Arctostaphylos (manzanita) and Ceanothus, as well as Adenostoma fasciculatum, Rhamnus californica and others. In this region, fire plays a relevant role in the ecosystem and the chaparral is closely associated with it. The abundance of serpentines shapes a substantial number of vegetation types. In the coastal strip, the sagebush formation with several Salvia species is also charactaristic (
In South America there is a region under similar Mediterranean climatic conditions in Central Chile, between 31 and 38°S. In this area thrive evergreen sclerophyllous forests with Cryptocarya alba, Jubaea chilensis, Lithraea caustica, Peumus boldus and Quillaja saponaria, which are replaced by a shrubland (matorral) when disturbance occurs, with Adesmia microphylla, Bahia ambrosioides, Fuchsia lycioides and other species (
The small Cape region in South Africa is covered with the shrubby evergreen vegetation called fynbos, which is comprised of sclerophyllous small-leaved scrubs of an extremely high variety of species, with high endemicity (Mucina and Rutherford 2006). This subbiome is adapted to extremely nutrient-poor soils and to frequent fires. With very few exceptions (Protea nitida), all the species of the fynbos are scrubby, many of them belonging to the Erica genus, as well as species of Proteaceae, Asteraceae, Rhamnaceae, Thymleaceae and Restionaceae.
Southwestern Australia is another of these “Mediterranean” regions having precipitations concentrated in the winter season and a low continentality (
5.b. Continental scrub and woodlands.
Related to the previous subbiome, when continentality increases due to altitude or distance from the oceans and winter temperature descends frequently below zero, sclerophyllous-microphyllous broadleaved woody plants decrease and there is an increase in conifers and deciduous tree species. Conifers belonging to Juniperus or Pinus genera form a vegetation of open woodland with a scrub covering the floor. There are several regions with this subbiome, all of them in the NH: western United States, Central Asian highlands and the Zagros range, as well as the highlands of the southern Atlas ranges in North Africa. In North America it is represented by the Pinyon-Juniper Woodland (
5c. Patagonian shrublands.
Due to the coincidence of particular geographic and orographic conditions, this subbiome is only found in the Argentinian Patagonia, on the eastern side of the southern Andean Range. It is a very unique unit from both the climatic and floristic-vegetation point of view. It is a region with pronounced summer aridity due to the rain shadow of the Andes combining with the southern westerlies coming off the Pacific. However, temperatures and precipitations are quite low, but the ombrothermic index (Io) is not so low to be considered as a true desert. This results in a cool and dry climate where summer is dry matching the aridiestival conditions in the broad sense. Due to the maritime influence, the annual temperature range is low. The vegetation is a grassy shrubland formed by species with clear drought adaptations such as spiny aphyllous or cushion dwarf shrubs. Some species are: Chuquiraga rosulata, C. avellanedae, Ephedra ochreata, Grindelia anethifolia, G. chiloensis, Malguraea tridens, Larrea ameghinoi, L. nitida, Mulinum spinosum, Nardophyllum bryoides, Nassauvia glomerulosa, Prosopis denudans, Prosopidastrum globosum, Retanilla patagonica, etc. Grasses are common, with species such as Festuca argentina, F. pallescens, Pappostipa humilis, Poa alopecurus etc. There are some species in common with the Central Chilean region, such as Lycium chilense or Mulinum spinosum, showing the climatic proximity of western Patagonia with central Chile (
BC. Temperate hypercontinental steppic ecozone
Low levels of precipitation throughout the year, accompanied with a high annual temperature variation range (continentality or BIO7 > 300), produce a type of vegetation often dominated by turf-grasses in which other growth forms are less abundant. The extreme cold of the winter combined with a not so low summer rainfall are the conditions of the steppes persisting in the central areas of the big continents of the NH. In North America, these occupy a vast extension in the center of the continent, where the Rocky Mountains buffer the influence of the Pacific Ocean and are separated from the east coast and the influence of the Atlantic Ocean by the wide rainy regions of the east and the Appalachians. Though some authors recognise similar biotic units in the SH like the Patagonian steppe and grasslands in South America (
6. Biome of the steppe.
The steppes form a zonal biome whose dominant vegetation is made up of grasses and grass-like plants that constitute the dominant element mixed with a large number of herbs and a mixture of chamaephytes. The main climatic characteristic of the steppe is continentality; that is, the high annual temperature range, which implies usually very cold winters. The precipitations are generally moderate or low, taking place mainly in summer; in winter they are scarce and are produced mainly in the form of snow. Usually, the summer coincides with the peak of rains, leading to no or moderate drought in the growing season. Thus, winters are dry and windy, while summers are relatively rainy except in the driest versions in which a certain summer aridity is manifested. Such a climate, a combination of a cold or very cold dry winter and a growing season with sufficient rainfall or moderately dry, cannot support the development of taller and more closed woody vegetation than that of a grassland or dwarf-shrubland (
6.a. Forest-steppe.
In Eurasia, this subbiome covers a large extent between eastern Central Europe and East Asia, occupying an intermediate spatial position between the closed forests (taiga or Temperate nemoral forests) and the treeless steppes, under transitional climatic conditions. It is formed by a typical pattern of mosaic of forest and grassland vegetation (
The most common trees in this subbiome are those present in the nearby forest areas, such as Betula pendula, B. pubescens, Pinus sylvestris, Populus tremula, Querus robur, Ulmus pumila, etc. In the eastern territories there are also Betula platyphylla, Larix sibirica and others. The grasslands are dominated by grasses such as Festuca valesiaca, Koeleria macrantha, Stipa capillata, S. pennata, S. tirsa and herbs such as Fragaria viridis and Filifolium sibiricum.
In North America, this subbiome can be roughly recognized in the tallgrass prairie that extends in a north-south strip in the eastern side of the biome as a transition to the temperate deciduous forest. Accordingly, this subbiome is moister than the following one, likely enabling it to bear a tree component. It has a scattered-tree overstory of Quercus marilandica and Q. stellata wth an understorey of Andropogon gerardii, Panicum virgatum, Schizachyrium scoparium, Sorghastrum nutans, etc. (
6.b. Grass-steppe.
When climatic conditions are more extreme, i.e. lower precipitations and colder and longer winters, the trees become scarce, or even disappear, and we have the true grass-steppe which form a grassy treeless landscape. It occupies the temperate areas subjected to higher continentality, both in North America and in Eurasia.
In central Eurasia this subbiome covers a large extent between the northern Black Sea and East Asia, in a fringe south to the forest-steppe. The southern fringe of this area usually has a drier climate and a “desert-steppe” is recognized locally. Species are numerous, many of them grasses but also other herbs. We can mention some as Agropyron cristatum, Allium strictum, Bothriochloa ischaemum, Cleistogenes squarrosa, Krascheninnikovia ceratoides, Salvia nutans, Sisymbrium polymorphum, Stipa glareosa, S. lessingiana, S. zalesskii, Veronica spicata, Scorzonera austriaca, etc. (
The North American grass-steppe zone comprises the so-called “short-grass prairie” and the “mixed-grass prairie” (
C. Xerocratic Domain of the arid climates
The most decisive climatic characteristic of this domain is the extreme aridity, which is mostly due to the predominance of permanent subtropical highs (
CA. Deserts and semi-deserts of arid regions ecozone
This is the only ecozone recognized in this domain.
7. Biome of the deserts and semi-deserts of arid regions
This is the only biome recognized in this ecozone.
7.a. Cold deserts and semi-deserts.
In central Asia, there is a vast arid territory from the eastern shores of the Caspian Sea to southern Mongolia and northeastern China, encompassing southern Kazakstan, vast portions of Uzbekistan and Turkmenistan, Dzungaria as well as the Taklamakan and the Gobi Deserts. Aridity, measured by Io, keeps usually below 1 but due to altitude in some areas exceptionally increases near to 2. This huge continuous arid territory is created by the rain decrease due to distance from the oceans and several mountain ranges rising around. It still receives scarce winter and spring precipitations of Atlantic origin in its western part, whereas its eastern part is under the influence of the monsoonal regime, receiving some rains from the Pacific in summer (
7.b. Temperate deserts and semi-deserts.
These occur in arid areas in which thermic seasons are clearly distinguished with a winter in contrast to a warm and hot summer.
The deserts and semi-deserts of western North America occupy the depression between the Rocky Mountains and the Sierra Nevada-Cascades range, which create a strong rain shadow between them. The Mohave Desert, dominated by Larrea tridentata (creosote bush) is included in this category, and in Mexico the Chihuahua desert, confined to the depression between Sierra Madre Occidental and Oriental, penetrating much to the south. In spite of its low latitude, this desert is relatively cool and rainy (Io between 1 and 2) due to its high altitude, with elevations mostly between 1100 m and 1500 m. The Neotropical flora is dominant with a high proportion of succulents such as Agave (A. lechuguilla), Yucca (Y. torreyi) and cacti species (
In Australia the temperate arid regions extend across the southern half of the central areas of the continent, bearing a semi-desert with a number of shrubby species such as Acacia aneura (mulga). There are also several species of spiny grasses of the genera Triodia (T. basedowii and others) and Plectrachne schinzii called spinifex grasses (
In the Old World, the temperate deserts have a large representation which includes the northern half of the Desert of the Sahara, the northern part of the Arabian Peninsula, Mesopotamia with Iraq, Jordan and Siria, as well as central Iran and Afghanistan, and western Pakistan. They are under extremely arid conditions with a marked thermic seasonality and rains falling in winter. Some relevant species are Aristida acutiflora, Calligonum polygonoides, C. comosum, Zilla spinosa and Calotropis procera, whereas in the western areas close to the Atlantic coasts, Euphorbia stem-succulents appear such as Euphorbia echinus (
In southwestern Africa there is an important region of aridity formed by the coastal Namib desert, the Karoo, the Succulent Karoo, Nama-Karoo and the Kalahari towards the inland (Mucina and Rutherford 2006). In general, the aridity is due to the dry air masses provided by the South Atlantic Subtropical High, which is a result of the descending arm of the Hadley cell in that area, in combination with the rain shadow created by the South African Great Escarpment range which dries up the SE humid trade winds from the Indian Ocean. The coastal Namib desert strip is influenced by the Benguela current in which cold waters favour the formation of frequent fogs. These areas are mostly included in the temperate deserts as they are influenced by the cool waters of the Benguela current in the coastal areas and the highlands in inland, which attenuate the high temperatures. The vegetation varies from the coastal desert where Aloe dichotoma is found, to the Succulent Karoo with great diversity and abundance of succulent Aizoaceae and Crassulaceae as well as Asteraceae and geophytes. Acacia mellifera subsp. detinens and Lycium cinereum inhabit more in inland districts.
7.c. Warm deserts and semi-deserts.
The arid regions within the tropical climatic latitudes, i.e. with a reduced thermic seasons, post equinox precipitations and expanding in lowlands, are included in this subbiome. In Tropical America there are two areas in which these deserts can be recognized, the Sonoran desert in North America and the Pacific Coastal Desert in South America. The Sonoran Desert occupies an area south of the Mojave Desert, from southern California and Arizona extending to the Mexican territories of coastal Sonora and most of Baja California. The vegetation is dominated by a high number of cacti as well as some Fouqueria (ocotillo) and Parkinsonia species (palo verde). The Pacific Coastal Desert occupies a narrow strip between the Andes range and the Pacific shores, from northern Peru to mid Chile spanning between 6° and 27°S latitude. This arid strip is a result of the rain shadow created by the high Andes against the southeastern trade winds, which is enhanced by the cold waters of the Humboldt stream. The vegetation is very sparse and there are communities of Tillandsia which survive by taking the humidity from the frequent coastal fogs (garúas), and some cacti (Neoraimondia, Armatocereus, etc.) in inland sectors (Galán de Mera 2004;
The southern Sahara desert is continued in the Arabian Peninsula and southern Iran and Pakistan until the Indian Sind-Thar. This vast strip is under tropical climatic influence and the rainfalls occur in the summer months. Some species of this territory are Acacia senegal, Aristida pungens, Cornulaca monacantha, Indigofera oblongifolia or Neurada procumbens (
The northern section of the western South African deserts, in the coastal regions of northern Namibia up to southern Angola, can be included in the category of the warm deserts. It is well known that Welwistchia mirabilis is a remarkable element in the flora of this area.
The tropical Australian desert occupies the northern fringe of the arid region of the continent where the rainfalls are received predominantly in summer (
D. Thermocratic Domain of the warm climates
This domain is extended through the lowland areas spanning approximately between both tropics, receiving the maximal amount of solar radiation and having a positive radiative balance. Thus, Pt is usually over 2000 units. Thermic seasons are not distinguishable in this tropical zone because the solar radiation falls on the earth almost vertically all year round, and there are at least two days each year in which the sun rays are perpendicular to the ground. Seasonality, when it exists, is due to yearly rainfall distribution. Precipitations can be continuous all year in the areas influenced by the ITCZ, or seasonal in the areas more separated from the lowest latitudes and subject to a monsoonal regime. In the last case, the rain season occurs in the “summer” (post-summer equinox) months. Vegetation is adaptad to these thermic and pluviometric circumstances and phenology does not follow the mandatory concentration of flower and fruit production of the temperate world but only the rain seasonal regime.
Mountains rising within this tropical zone reproduce tropical versions of the biomes of the mesocratic and cryocratic domains in their vegetation belts, which are formed partially by local lineages combined with others migrated from higher latitudes in different climatic episodes. In our classification, these mountain formations will be explained within the biomes of the mesocratic and cryocratic domains as the lower temperatures of the higher elevations determine substantial convergences with them in terms of climate, growth forms and often biogeographical relationships. Thus, we will consider warm (tropical) biomes stricto sensu to those of the lowlands up to 1000–1500 m asl (infra and thermo).
DA. Tropical pluviseasonal ecozone
As indicated above, in the intertropical latitudes the major part of the rainfalls occur in the post-summer equinox (summer) months and if this entails a wet and a dry season, so we will find an important set of adaptations to the drought. In many areas trees and shrubs become deciduous or semideciduous to prevent water loss. Woody plants often become spiny to prevent herbivory and the woody formations adopt a low density leaving room for many other smaller plants, like grasses and shrubs. Sclerophylly and evegreeness appears when soils are poor in nutrients.
8. Biome of the tropical pluviseasonal forests and woodlands.
In general, the vegetation is adapted to pluvial seasonality and in the dry season there is a complete or partial shedding of the leaves. In some areas where soils are very poor, woody plants become sclerophyllous and evergreen, as in the Brasilian cerrado (
8.a. Tropical xeric woodlands and shrublands
This subbiome represents the transition from the warm desert (7.c) to the pluviseasonal areas with a longer rainy season and abundant precipitations, which in many cases follows a flat gradient which leaves substantial areas of dubious qualification. The seasonality of the precipitation becomes strongly unbalanced in favor of the dry season, which covers over 8 months of the year and with mostly arid and sub-arid ombrotypes. This results in a shrubby and spiny vegetation, often with many succulents.
In Africa, it is greatly extended in the Sahel, between Senegal and Sudan, in the western African Horn (Somalia and Masai areas) and in the transitional areas to the deserts of the southern part of the continent.
In the vast territories of the Sahel the vegetation comprises thorny shrubs or trees, mainly acacias, such as Acacia nilotica, A. tortilis, Senegalia laeta and S. senegal. Other tree species are Balanites aegyptiaca, Boscia senegalensis, Comminphora africana or Faidherbia albida. The grass layer is formed by annuals such as Aristida stipoides, Cenchrus biflorus, Panicum turgidum and Schoenefeldia gracilis, which dry up in the dry season when the trees and shrubs shed their leaves (
In the southern African territories covered by this subbiome, Acacia erioloba, Colophospermum mopane and Terminalia sericea with grasses of Aristida, Eragrostis or Panicum, are frequent (Mucina and Rutherford 2006).
In tropical America, it is dominant in the southern Chaco and Monte in Argentina and nerby countries, the Guajira in Venezuela and the Caatinga in Brazil. The Guajira-Maracaibo in northern Venezuela and Colombia occupies an area around the gulf of Maracaibo, with some relevant species including Castela erecta, Cercidium praecox, Cesalpinia coriaria, Gyrocarpus americanus, Haematoxylon brasiletto, Myrospermum frutescenes, Prosopis juliflora and the cacti Acanthocereus columbianus, Cephalocereus russelianus and Ritterocereus griseus (
Northeastern Brazil is occupied by the caatinga, a dry deciduous spiny vegetation rich in legumes with species such as Auxemma oncocalyx, Caryocar cuneatum, Caesalpinia bracteosa, Maytenus rigida, Mimosa caesalpiniifolia, Piptadenia viridifolia, Ziziphus joazeiro and others (
In India, this subbiome ranges over a large area around the west of the Deccan plateau, including east of Rajasthan, south-west Punjab, Haryana, Gujarat and parts of Uttar Pradesh, Madhya Pradesh, Maharastra, Telangana and Andra Pradesh. The species of Indian distribution such as Acacia planiforns or Azadirachta indica, mix with others of broader range within this subbiome including Acacia nilotica, Butea monosperma or Prosopis cineraria (
In Australia, the distribution of the subbiome occupies a strip in the central northern regions, between the pluviseasonal forest and the warm desert (
8.b. Tropical pluviseasonal forests
This subbiome corresponds to the mesic version of the biome and is formed basically by tree-dominated vegetation, often with a grassy (savanna) or scrubby understorey. Climatic conditions entail a dry season between 1–2 to 6–7 months, where double temperatures are higher than precipitation.
In tropical Asia and Malesia, the vast areas occupying most of the Indian subcontinent, as well as territories in Indochina and Malesia, are subjected to a tropical climate with a dry season, and a deciduous forest develops which corresponds to this biome. In it species such as Anisoptera thurifera, Anogeissus latifolia, Areca catechu, Azadirachta indica, Bombax ceiba, Butea monosperma, Dalbergia sissoo, Diospyros melanoxylon, Ficus religiosa, Garuga floribunda, Haldina cordifolia, Intsia bijuga, Madhuca longifolia, Phyllanthus emblica, Protium macgregorii, Pterocarpus indicus, Schleichera oleosa, Senegalia catechu, Shorea robusta, Tectona grandis and others can be found (
In tropical America, this biome is widely represented all over the continent, from Mexico to the south American Paraná-Paraguay river basin, encompassing a range of more humid to more xeric variants. There are a couple of well defined regions which are occupied by this biome in their particular versions: In Mesoamerica (southern Mexico and Central America, as well as in the Caribbean islands), there are important areas occupied by this biome, particularly on the Pacific side, with deciduous trees such as Swietenia humilis, Bombacopsis quinatum, Cedrela fissilis, C. mexicana, C. odorata, C. salvadorensis, Ceiba aesculifolia, Dalbergia retusa; many legumes including Enterolobium cyclocarpum, Hymenaea courbaril, Cassia grandis, Platymiscium dimorphandrum, Samanea saman; and the palms Acrocomia aculeata, A. crispa, etc. and succulents (Cactaceae and Agavaceae) (
The area of the Orinoco watershed shared by Colombia and Venezuela, known as the Llanos, is a flat region which is influenced by extensive floods (Pantanal). In the non flooded areas a woody vegetation of evergreen semideciduous trees is established, with tropical American species such as Bowdchia virgilioides, Brysonima crassifolia or Curatella americana, which combine with other endemics such as Borreria aristeguietana, Duguetia riberensis or Randia venezuelensis (
The Brazilian cerrado occurs widely across central Brazil (
The Chaco is a vast region extending along the plain east of the central Andes through southwestern Brazil, most of Bolivia and Paraguay and northern Argentina. It is occupied by a deciduous forest in which occur species such as Aspidosperma quebracho-blanco, Ceiba speciosa, Piptadeniopsis lomentifera, Prosopis ruscifolia, Schinopsis balansae, S. cornuta, Sideroxylon obtusifolium, Ziziphus mistol and others, with some grasses such as Cenchrus pilcomayensis and Elionorus muticus (
In tropical Africa this subbiome occupies its largest extent, surrounding the tropical rain forest biome area and constrained by the 8.a subbiome (
In Australia this subbiome occupies a vast area encompassing the northernmost regions of the Continent such as the Kimberly Plateau, the area of Darwin and the Carpentaria Plains (south of the Gulf of the same name), most of the Cape York peninsula and other areas in the northeast. It occupies the landscapes between the shores of the Timor and Arafura seas and the xeric inland regions of the continent. The vegetation is dominated by woodlands formed by evergreen and hard-leaved eucalypts. This sclerophyllous evergreen life form is consistent with the poverty of the soils widespread on the Australian continent. Some important trees are Acacia shyrleyi, Corymbia confertiflora, C. grandifolia, C. polycarpa, Eucalyptus brevifolia, E. dichromophloia, E. grandifolia, E. miniata, E. phoenicea, E. pruinosa, E. tectifica, E. tetrodonta, etc. Some other woody plants are Acacia humifusa, A. mountfordiae, Hakea arborescens, Grevillea pteridifola or Lysiphyllum cunninghamii (
DB. Tropical pluvial ecozone
In the tropical areas where rainfall persists all year round (Rainfall seasonality BIO15 < 60) in a sufficient intensity and frequency so that no or a very brief (1 month) dry season can be recognized, the tropical pluvial ecozone is established. Temperatures are high and present low daily and yearly oscillations, and precipitations rarely fall below 2000 mm per year and occur usually at zenital showers in the afternoon. The soils undergo an intense leaching leading to the formation of laterites.
9.Biome of the tropical rain forests.
This biome occurs where the tropical warm and moist forest thrives, usually called tropical rainforest or pluviisilva. It has a high number of vascular plant species, including trees, which bear particular adaptations to this favorable environment: thin barks, leaves with entire margins, gutation organs and other well documented features of this tropical rainforest (
9.a Tropical rain forests
This is the only subbiome recognized withis this biome.
In Tropical America, there are several areas occupied by this biome-subbiome:
In Central- and Mesoamerica, this biome-subbiome develops mostly in the Caribbean side, occupying the entire isthmus of Panamá and peripheral areas in the Antilles, including the southern half of the Florida peninsula.
The most important area is the vast Amazonian plain (the hylea amazonica of
Another characteristic region is the Darién-Chocó in the Pacific coastal strip from southern Panamá to Colombia and Ecuador, which receives abundant precipitations from the east. The tropical rain forest of this area has many endemics and there are species such as Billia columbiana, Exarata chocoensis, Guettarda crispiflora subsp. sabiceoides, Humiriastrum procerum, Virola dixonii and others (Rangel 1997).
The low altitudes of the Atlantic coastal fringe of Brazil, spanning between Bahia and Santa Catarina are covered by this biome-subbiome. It is represented by the Mata Atlântica or Floresta Atlântica, a highly diverse tropical forest maintained by the moist trade winds of the south Atlantic, which provide abundant rainfall all year. Due to the higher latitudes and elevations in the mountains of the coastal range, the thermic regime has lower temperatures than in the Amazonian river basin. The natural forest is extremely rich in species, particularly epiphytes with many bromeliads, making each tree resemble a true garden in miniature (
The Guineo-Congolian region is the most important of tropical Africa, encompassing the coastal strip of the gulf of Guinea from Sierra Leone to Nigeria and entering deep into the African continent in the Congo watershed until the chain of mountains and lakes crossing east Africa, including large parts of Cameroon, Gabon and Ecuatorial Guinea (
The Madagascar eastern coast is homologous to the Mata Atlântica region of Brazil. The flora is mainly African with a high number of endemics, among them Ravenala madagascariensis. (
The Indo-Malesian region is a large territory encompassing south China, the Philippines, the islands of Malesia (Java, Sumatra, Borneo, Celebes, Moluccas, etc.), the peninsula of Malacca, parts of Indochina and the gulf of Bengal to the piedmonts of the eastern Himalayas. For Malesia we can mention some species such as Artocarpus elasticus, Macaranga hispida, Nephelium lappaceum, Palaquium rostratum, Platea excelsa, Prunus arborea, Shorea leprosula, Trevesia sundaica etc. (
The Malabar-Ceylon region lies along the southwestern coast of the Indian subcontinent, at the piedmonts of the Gaths range and the western half of the Sri Lanka (Ceylon) island.
The Pacific Oceanic region encompass the numerous islands widespread over the Pacific Ocean. The flora and vegetation of this inmense number of islands is full of endemic species having originated from neighbouring continental Godwanic islands (basically New Zealand and New Caledonia) and from tropical East Asia across the Malesian archipelagos. There are representatives of gymnosperms of the genera Agathis, Dacrydium or Podocarpus and some species of a more general distribution are Calophyllum neoebudicum, Canarium vitiense, several species of Metrosideros such as M. collina, among many others (
The Papuan-Australian region includes the island of New Guinea and the northeastern extreme of the Australian continent. In Australia only a narrow area in Queensland can be assigned to this biome, in the eastern coastal strip of the Cape York Peninsula, bearing similarities with the near island of New Guinea. Some species are Agathis microstachya, Balanophora fungosa, Balanops australiana, Cordyline cannifolia, Macaranga inamoena, Musa banksia, Pandanus monticola, Syzygium wilsonii and others (
Results and discussion
In this section, we will provide comment on the climatic characterization of the considered biotic units (domains, ecozones, biomes and subbiomes) by means of data from the 616 selected locations.
Ordination diagrams
PCA ordination was performed for the selected locations and plotted along axis 1 and 2, which encompasses most of the variability of the data (40.1% for axis 1 and 29.7% for axis 2, Figure
Importance of the selected variables in the PCA ordination axis 1 vs. 2. BIO1 = Annual Mean Temperature (T); BIO2 = Mean Diurnal Range (Mean of monthly (max temp - min temp)); BIO3 = Isothermality (BIO2/BIO7) (×100); BIO5 = Max Temperature of Warmest Month; BIO6 = Min Temperature of Coldest Month; BIO7 = Temperature Annual Range (BIO5–BIO6); BIO8 = Mean Temperature of Wettest Quarter; BIO9 = Mean Temperature of Driest Quarter; BIO10 = Mean Temperature of Warmest Quarter; BIO11 = Mean Temperature of Coldest Quarter; BIO12 = Annual Precipitation (P); BIO15 = Precipitation Seasonality (Coefficient of Variation); BIO16 = Precipitation of Wettest Quarter; BIO17 = Precipitation of Driest Quarter; BIO18 = Precipitation of Warmest Quarter; BIO19 = Precipitation of Coldest Quarter; nfd = number of frost days; dws = drought of the warm season (0BIO18/BIO10); Tp = positive temperature (Rivas-Martínez); Pp = positive precipitation (Rivas-Martínez); Io = (Pp/Tp) × 10 Ombrothermic index by Rivas-Martínez; CI = Coldness Index (Kira).
In domain B, biome 4 occupies a central position (Figure
In Figure
In Figure
Boxplots
In this section, the climatic parameters used for the PCA are examined separately in order to see the differences between the domains, ecozones, biomes and subbiomes (boxplots available in Suppl. material
Concerning the domains, the thermometric parameters indicate a clear separation of the four domains in terms of mean annual temperature (BIO1, 6, 8, 9, 10, 11, CI, nfd, tp) and of isothermality (BIO3). The summer warmth intensity (BIO5) separates also the four domains with a clear increase in the case of the xerocratic deserts and arid areas. The temperature annual range (BIO7) in these large units only separates the thermocratic domain which have the lowest scores. The pluviometric parameters (BIO12, 13, 14, 16, 17, 18, Io, Pp) separate clearly the xerocratic domain from the other three. Other parameters (BIO15) are not discriminated at this level.
At the biome level, thermometric parameters (BIO1, 6, 8, 9, 10, 11, CI, nfd, Tp), separate along a gradient from the colder ones to the warmest, but the steppes (6) fall down almost to the level of the boreal cold forest (2). The mean diurnal range (BIO2) also reveals high scores for the steppes and the lowest in the tropical rain forest (9), quite opposite to isothermality (BIO3). The summer warmth intensity is maximal in deserts and minimal in the tundra (1), while the temperature annual range (BIO7) is maximal in the steppe and minimal in the tropical rain forest (see also Figure
In the case of the subbiomes, we see that the altitude parameter is relevant because it reveals the orographic condition of some of these, specifically 1.b, 1.c, 2.b, 4.c and 5.b. The thermometric parameters (BIO1, 6, 8, 9, 10, 11, CI, nfd, Tp) follow the general patterns, as do the biomes but also separating the orophytic subbiomes. In the case of biome 4, 4.c appears to be warmer than 4.b and that is due to the higher latitudes of 4.b. BIO1 and Tp show a quite similar pattern, with the only difference being that in the positive temperature the boreal forest subbiome (2.a) records higher values in Tp. In BIO2, diurnal temperature ranges are lowest in both extremes and highest in the strongly seasonal subbiomes such as the deserts (7.a, 7.b and 7.c), the steppe (6.b) and the continental aridiestival temperate biome (5.b). Tp across the world is represented in Figure
Pluviometric values are represented in BIO12, where the deserts (7.a, 7.b and 7.c) are the driest, followed by the Patagonian shrubland (5.c). On the opposite side, the tropical rain forest (9.a), the tropical cryoro tundras (1.c), the tropical montane forests (4.b) and the conifer coastal forest (4.c) receive the highest amount of precipitation, and these results are similar to those of Pp. Comparing the precipitation in the driest (BIO17) and the wettest (BIO16) month and quarter reveals that the difference between them is higher in the tropical cryoro tundra (1.c), in the tropical montane forests (4.b) and in the tropical pluviseasonal biome (8), where the wet season receives more rain. Precipitation seasonality (BIO15) is highest in the warm desert (7.c) and in the tropical pluviseasonal biome (8), being low in the Patagonian shrubland (5.c). The precipitation in the warmest quarter (BIO18), as an estimation of the intensity of the seasonal drought, is highest in the deserts (7) but also remarkable in the temperate aridiestival (Mediterranean s.l.) biome (5). Precipitation in the coldest quarter (BIO19) shows high scores for the tropical montane forests (4.b).
Other indices have also been used for the analysis. The CI (Kira’s cold index) basically reproduces the pattern shown by BIO6 and BIO11 in relation to the temperatures of the coldest period.
The drought of the warm season (dws) distinguishes clearly the deserts (7.a, 7.b and 7.c) in a comparable position to the temperate aridiestival biome (5.a, 5.b and 5.c), highlighting the summer drought of the latter.
Io is a strong indicator of aridity in the xerocratic biomes (7), in the grass-steppe (6b), the Patagonian shrubland (5c), the tropical xeric shrublands and woodlands (8a), the temperate deciduous forests (3), and in the continental temperate aridiestival biome (5.b). Opposite to it, the tundral biome (1) and the tropical montane cloud lauroid and conifer evergreen forest (4c) reach the highest values. Figure
Conclusions
Despite the great floristic diversity that exists across the different regions of the planet, a comprehensive bioclimatic scheme is presented for all the world’s vegetation, which has been categorized into a small number of units with their distributions explained by climatic factors. The most notable conclusions that can be drawn from the application of this system can be summarized as follows:
- There is homology between the latitudinal and altitudinal zoning of the ecosystems through this biome system.
- There is asymmetry between the two hemispheres: Biomes 2 and 3 are overwhelmingly more abundant and diverse in the NH and biome 6 and subbiome 5.b are exclusive of it. This is a consequence of its higher continentality in the more extensive land masses covering extratropical latitudes. Conversely, biome 4 is more widely distributed in the SH.
- There is asymmetry in the occurrence and latitudinal distribution of biomes along the continental masses: the western sides of continents are subjected to drought caused by subtropical high pressure systems determining the existence of biomes 7 and 5, which are lacking in the eastern sides. These biomes associated with aridity expand inland in the case of Eurasia due to the enormity of that continent.
- For the climatic characterization of zonal biotic units, large sets of climatic parameters such as that provided by CHELSA should be used.
- The climatic characterization does not provide sharp boundaries, as can be expected due to the transitional nature of the biotic units.
Data availability
Used data are available in the Suppl. material
Author contributions
J.L. planned the survey, conducted the analysis and wrote the manuscript, G.N.-S. provided information on the tropical world, his vision on the typology in South America and revised the manuscript, D.V. performed all analyses, prepared the figures, provided his insight and knowledge on the steppes, and revised the manuscript.
Acknowledgements
Funds from the project IT936-16 of the Basque Government have been used in this survey.
References
- Aerts R, Overtveld KV, Haile M, Hermy M, Deckers J, Muys B (2006) Species composition and diversity of small Afromontane forest fragments in northern Ethiopia. Plant Ecology 187: 127–142. https://doi.org/10.1007/s11258-006-9137-0
- Alaback PB (1991) Comparative ecology of temperate rainforests of the Americas along analogous climatic gradients. Revista Chilena de Historia Natural 64: 399–412.
- Bailey RG (1998) Ecoregions map of North America: Explanatory note. [Misc. Publ. 1548]. USDA Forest Service. Washington DC, US, 10 pp.
- Barbour MG, Keeler-Wolf T, Schoenherr AA (2007) Terrestrial vegetation of California. 3rd University of California Press, Berkeley, Los Angeles, London, US, 713 pp. https://doi.org/10.1525/9780520933361
- Beadle NCW (1981) The vegetation of Australia. Gustav Fischer, Stuttgart, DE. https://doi.org/10.1007/978-94-009-8629-9_23
- Beard J (1975) The vegetation survey of Western Australia. Vegetatio 30: 179–187. https://doi.org/10.1007/BF02389706
- Beard J (1990) Plant life of Western Australia. Kangaroo Press, Kenthurst, 310 pp.
- Beard J, Chapman AR, Gioia P (2000) Species richness and endemism in the Western Australian flora. Journal of Biogeography 27: 1257–1268. https://doi.org/10.1046/j.1365-2699.2000.00509.x
- Bliss LC, Courtin GM, Pattie DL, Riewe RR, Whitfield DWA, Widden P (1973) Arctic Tundra Ecosystems. Annual Review of Ecology and Systematics 4: 359–399. https://doi.org/10.1146/annurev.es.04.110173.002043
- Bond WJ (2005) Large parts of the world are brown or black: A different view on the “Green World” hypothesis. Journal of Vegetation Science 16: 261–266. https://doi.org/10.1658/1100-9233(2005)016[0261:LPOTWA]2.0.CO;2
- Botti D (2018) A phytoclimatic map of Europe. Cybergeo: European Journal of Geography [Online], Environment, Nature, Landscape, document 867. https://doi.org/10.4000/cybergeo.29495
- Box EO (1981) Macroclimate and Plant Forms: An Introduction to Predictive Modeling in Phytogeography. Tasks for Vegetation Science, vol. 1. Dr. W. Junk BV. The Hague, NL, 58 pp. https://doi.org/10.1007/978-94-009-8680-0_1
- Box EO, Fujiwara K (2005) Vegetation types and their broad-scale distribution. In: van der Maarel E, Franklin J (Eds) Vegetation Ecology. Blackwell, Oxford, UK, 106–128.
- Box EO, Fujiwara K (2013) Vegetation types and their broad-scale distribution. In: van der Maarel E (Ed.) Vegetation Ecology 2nd edition. Wiley and Sons, Oxford, UK, 455–485. https://doi.org/10.1002/9781118452592.ch15
- Box EO, Fujiwara K (2015) Introduction: why warm-temperate deciduous forests? In: Box EO, Fujiwara K (Eds) Warm-Temperate Deciduous Forests around the Northern Hemisphere. Geobotany Studies. Springer, 1–5. https://doi.org/10.1007/978-3-319-01261-2_1
- Breckle S-W (2002) Walter’s vegetation of the Earth. The ecological systems of the Geo-Biosphere. 4th Ed. Springer, Berlin, DE, 527 pp.
- Brochmann C, Brysting AK (2008) The Arctic – an evolutionary freezer? Plant Ecology and Diversity 1: 181–195. https://doi.org/10.1080/17550870802331904
- Brockmann-Jerosch H, Rübel E (1912) Die Einteilung der Pflanzengesellschaften nach ökologisch-physiognomischen Gesichtspunkten. Wilhelm Engelmann Verlag, Leipzig, DE, 72 pp.
- Buot IE (2002) Vegetation types of mount Akiki, northern Luzon, Philippines. Flora Malesiana Bulletin 13(2): 154–156.
- Cabrera AL (1971) Fitogeografía de la República Argentina. Boletín de la Sociedad Argentina de Botánica 14(1/2): 1–42.
- Christensen NL (1988) Vegetation of the southeastern Coastal Plain. In: Barbour MG, Billings WD (Eds) North American terrestrial vegetation. Cambridge University Press, 317–363.
- Clements FE (1917) The development and structure of biotic communities. The Journal of Ecology 5: 120–121.
- Comes HP, Kadereit JW (2003) Spatial and temporal patterns in the evolution of the flora of the European Alpine System. Taxon 52: 451–462 https://doi.org/10.2307/3647382.
- Davis MB (1983) Quaternary history of deciduous forests of Eastern Noth America and Europe. Annals of the Missouri Botanical Garden 70: 550–563. https://doi.org/10.2307/2992086
- Delgado LA, Castellanos H, Rodríguez M (2009) Vegetación. In: Señaris CJ, Dew D, Lasso C (Eds) Biodiversidad del Parque Nacional de la Canaima. Bases técnicas para la conservación de la Guayana Venezolana, 39–73.
- Edwards ID, Payton RW, Proctor J, Riswan R (1990) Altitudinal zonation of the rain forests in the Manusela National Park, Seram, Maluku, Indonesia. In: Baas P, Kalkmaan K, Geesink R (Eds) The plant diversity of Malesia. Kluwer, Dordrecht, NL, 161–175. https://doi.org/10.1007/978-94-009-2107-8_15
- Ellis EC (2020) Anthromes. In: Goldstein MI, Della Sala DA (Eds) Encyclopedia of the world’s biomes, 5–10. https://doi.org/10.1016/B978-0-12-409548-9.12494-7
- Emberger L (1955) Une classification biogéographique des climats. Recueil des Travaux des Laboratoires de Botanique, Géologie et Zoologie de la Faculté des Sciences de L’Université de Montpellier, Série Botanique 7: 3–43.
- Enright NJ, Miller BP, Akther R (2005) Desert vegetation and vegetation-environment relationships in Kirthar National Park, Sindh, Pakistan. Journal of Arid Environments 61: 397–418. https://doi.org/10.1016/j.jaridenv.2004.09.009
- Erdos L, Ambarli D, Anenkhonov OA, Bátori Z, Cserhalmi D, Kiss M, Kröel-Dulay G, Liu H, Magnes M, … Török P (2018) The edge of two worlds: A new review and synthesis on Eurasian forest-steppes. Applied Vegetation Science 21: 345–362. https://doi.org/10.1111/avsc.12382
- Faber-Langendoen D, Keeler-Wolf T, Meidinger D, Josse C, Weakley A, Tart D, Navarro G, Hoagland B, Ponomarenko S, … Helmer E (2016) Classification and Description of World Formation Types. Gen. Tech. Rep. RMRS-GTR-346. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, 222 pp. https://doi.org/10.2737/RMRS-GTR-346
- Fernández Calzado R, Molero Mesa J (2011) The cartography of vegetation in the cryoromediterranean belt of Sierra Nevada: a tool for biodiversity conservation. Lazaroa 32: 101–115.
- Fernández-González F (1989) Bioclimatología. In: Izco J (Ed.) Botánica. Mc Graw-Hill Interamericana, 607–682.
- Fernández-Palacios JM, Arévalo JR, Balguerías E, Barone R, de Nascimento L, Elias RB, Delgado JD, Fernández-Lugo S, Méndez J, … Otto R (2017) La Laurisilva. Canarias, Madeira y Azores. Macaronesia Editorial, Santa Cruz de Tenerife, ES, 417 pp. http://hdl.handle.net/10553/55513
- Franklin JF (1988) The Pacific Northwest. In: Barbour MG, Billings WD (Eds) North American terrestrial vegetation. Cambridge University Press, Cambridge, UK, 103–130.
- Galán de Mera A (2005) Clasificación fitosociológica de la vegetación de la región del Caribe y América del Sur. Arnaldoa 12: 86–111.
- Galán de Mera A, Baldeón S, Beltrán H, Benavente M, Gómez J (2004) Datos sobre la vegetación del centro del Perú. Acta Botanica Malacitana 29: 89–115. https://doi.org/10.24310/abm.v29i0.7227
- Galán de Mera A, González A, Morales R, Oltra B, Vicente Orellana JA (2006) Datos sobre la vegetación de los Llanos Occidentales del Orinoco (Venezuela). Acta Botanica Malacitana 31: 97–129. https://doi.org/10.24310/abm.v31i31.7124
- Galán de Mera A, Linares Perea E, Campos de la Cruz J, Vicente Orellana JA (2009) Nuevas observaciones sobre la vegetación del sur del Perú. Del desierto costero al altiplano. Acta Botanica Malacitana 34: 107–144. https://doi.org/10.24310/abm.v34i0.6904
- González Medrano F (2004) Las comunidades vegetales de México. 2ª ed. Secr. de Medio Ambiente y Recursos Naturales. Instituto Nacional de Ecología. México D. F., MX, 82 pp.
- Grau J (1992) Clima y distribución geográfica de la Flora de Chile. In: Grau J, Zizka G (Eds) Flora silvestre de Chile. Sonderheft 19, Palmengarten, Frankfurt am Main, DE, 11–24.
- Greller A (1988) Deciduous forests. In: Barbour MG, Billings WD (Eds) North American terrestrial vegetation. Cambridge University Press, Cambridge, UK, 287–316.
- Greller AM, Wijesundara DSA, Jayasuriya HM (2016) Classification of lower montane evergreen forests in southern India and Sri Lanka. In: Box EO (Ed.) Vegetation structure and functional multiple spatial, temporal and conceptual scales. Geobotany Studies. Basics, methods and case studies, Springer, Cham, CH, 169–213. https://doi.org/10.1007/978-3-319-21452-8_6
- Gressitt JL [Ed.] (1982) Biogeography and ecology of New Guinea. Dr. Junk, Publ. The Hague, NL, 981 pp. https://doi.org/10.1007/978-94-009-8632-9
- Grishin SY (1995) The Boreal forests of north-eastern Eurasia. Vegetatio 121: 11–21. https://doi.org/10.1007/BF00044668
- Grubb PJ, Bellingham PJ, Kohyama TS, Piper FI, Valido A (2013) Disturbance regimes, gap-demanding trees and seed mass related to tree height in warm temperate rain forests worldwide. Biological Reviews 88: 701–744. https://doi.org/10.1111/brv.12029
- Guijarro JA (2019) Climatol: Climate Tools (Series Homogenization and Derived Products). R package version 3.1.2. https://CRAN.R-project.org/package=climatol
- Hijmans RJ (2020) Raster: Geographic Data Analysis and Modeling. R package version 3.4-5. https://CRAN.R-project.org/package=raster
- Holdridge LR (1967) Life zone ecology. Tropical Science Center, San José, CR, 206 pp.
- Hopper SD (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322: 49–86. https://doi.org/10.1007/s11104-009-0068-0
- Huguet del Villar E (1929) Geobotánica. Ed. Labor, Barcelona, ES, 339 pp.
- Humboldt A von (1806) Ideen zu einer Physiognomik der Gewächse. Cotta, Stuttgart. [English translation by E C Otté and HG Bohn, as Ideas for a physiognomy of plants]
- Humboldt A von (1817) De distributione geographica plantarum secundum coeli temperiem et altitudinem montium, prolegomena. Libraria Graeco-Latino-Germanica, Paris, FR, 247 pp.
- Humboldt A von, Bonpland A (1805) Essai sur la géographie des plantes accompagné d’un tableau physique des régions équinoxiales, fondé sur des mesures exécutées, depuis la dixième degré de latitude boréale jusqu’au dixième degre de latitude australe, pendant les années 1799, 1800, 1801, 1802 et 1803. Levraut Schoell et Co. Paris, FR. https://doi.org/10.5962/bhl.title.9309
- Hunter JT, Franklin SB, Luxton S, Loidi J (2021) Terrestrial biomes: a conceptual review. Vegetation Classification and Survey 2: 73–85. https://doi.org/10.3897/VCS/2021/61463
- Hurka H, Friesen N, Bernhardt K-G, Neuffer B, Smirnov SV (2019) The Eurasian steppe belt: Status quo, origin and evolutionary history. Turczaninowia 22(3): 5–71. https://doi.org/10.14258/turczaninowia.22.3.1
- Hytteborn H, Maslov AA, Nazimova DI, Rysin LP (2005) Boreal forests of Eurasia. In: Andersson F (Ed.) Coniferous forests. Ecosysems of the world 6. Elsevier, Amsterdam, NL, 23–99.
- Jahn G (1991) Temperate deciduous forests of Europe. In: Röhrig E, Ulrich B (Eds) Temperate deciduous forests. Ecosystems of the World 7. Ed. D Goodall. Elsevier, Amsterdam, NL, 377–502.
- Josse C (2014) Macrogroups of South America. International Vegetation Classification Standard. NatureServe Biotics.
- Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder P, Kessler M (2017) Climatologies at high resolution for the Earth land surface areas. Scientific Data 4: 170122. https://doi.org/10.1038/sdata.2017.122
- Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder P, Kessler M (2018) Data from: Climatologies at high resolution for the earth’s land surface areas. Dryad Digital Repository. https://doi.org/10.1038/sdata.2017.122
- Keith DA, Ferrer-Paris JR, Nicholson E, Kingsford RT [Eds] (2020) IUCN Global Ecosystem Typology 2.0. Descriptive profiles for biomes and ecosystem functional groups. Gland, Switzerland, IUCN, 170 pp. https://doi.org/10.2305/IUCN.CH.2020.13.en
- Kira T (1977) A climatological interpretation of the Japanese vegetation zones. In: Miyawaki A, Tüxen R (Eds) Vegetation science and environmental protection. Maruzen Co. Ltd., Tokyo, JP, 21–30.
- Kira T (1991) Forest Ecosystems of East and Southeast Asia in a Global Perspective. Ecological Research 6: 185–200. https://doi.org/10.1007/BF02347161
- Kitayama K (1992) An altitudinal transect study of the vegetation on Mount Kinabalu, Borneo. Vegetatio 102: 149–171. https://doi.org/10.1007/BF00044731
- Klein DR (1970) Tundra ranges north of the Boreal Forest. 22th Annual Meeting of the American Society of Range Management, Calgary, CA, 1–14. https://doi.org/10.2307/3896000
- Köppen W, Geiger R (1954) Klima der Erde (map). Justus Perthes, Darmstadt, DE.
- Körner C (1995) Alpine Plant Diversity: A Global Survey and Functional Interpretations. In: Charpin D, Körner C (Eds) Arctic and Alpine Biodiversity: Patterns, Causes and Ecosystem Consequences [Ecological Studies 113]. Springer, Cham, CH, 45–62. https://doi.org/10.1007/978-3-642-78966-3_4
- Latham RE, Ricklefs RE (1993) Continental comparisons of temperate-zone tree species diversity. In: Ricklefs RE, Schluter D (Eds) Species Diversity in Ecological Communities: Historical and Geographical Perspectives. University of Chicago Press, Chicago, US, 294–317.
- Lavrenko EM (1970) Provincial’noe razdelenie Central’noaziatskoj podoblasti Stepnoj oblasti Evrazii [Division of the Central Asian subregion of the steppe region of Eurasia]. Bot. Zhurn. (Moscow and Leningrad) 55: 1734–1741. [In Russian]
- León RJC, Bran D, Collantes M, Paruelo JM, Soriano A (1998) Grandes unidades de vegetación de la Patagonia extra andina. Ecología Austral 8: 125–144.
- Liu C, Qiao X, Guo K, Zhao L, Qigmin Pan Q (2022) Vegetation classification of Stipa steppes in China, with reference to the International Vegetation Classification. Vegetation Classification and Survey 3: 121–144. https://doi.org/10.3897/VCS.72875
- Logan RF (1968) Causes, climates and distribution of deserts. In: Brown GW (Ed.) Desert Biology. Vol 1. Academic Press, New York, US, 21–50. https://doi.org/10.1016/B978-1-4831-9868-2.50010-4
- Loidi J (2021) Dynamic-catenal vegetation mapping as a tool for ecological restoration and conservation policy. In: Pedrotti F, Box EO (Eds) Tools for landscape-scale Geobotany and conservation. Springer, Cham, CH, 37–64. https://doi.org/10.1007/978-3-030-74950-7_4
- Loidi J, Chytrý M, Jiménez-Alfaro B, Alessi N, Biurrun I, Campos JA, Carni A, Fernández-Pascual E, Font Castell X, … Marcenò C (2021) Life-form diversity across temperate deciduous forests of Western Eurasia: a different story in the understory. Journal of Biogeography 48: 2932–2945. https://doi.org/10.1111/jbi.14254
- Loidi J, Fernández-González F (2012) Potential Natural Vegetation: reburying or reboring? Journal of Vegetation Science 23: 596–604. https://doi.org/10.1111/j.1654-1103.2012.01387.x
- Loidi J, Marcenò C (2022) The Temperate Deciduous Forests of the Northern Hemisphere. A review. Mediterranean Botany 43: e75527. https://doi.org/10.5209/mbot.75527
- Losa MT, Rivas Goday S, Muñoz Medina JM (1974) Tratado elemental de Botánica Descriptiva. Vol. II Fanerogamia. 5th Ed. Granada, ES.
- Lozada JR, Soriano P, Costa M (2014) Relaciones suelo-vegetación en una toposecuencia del Escudo Guayanés, Venezuela. Revista de Biología Tropical 62: 385–401. https://doi.org/10.15517/rbt.v62i1.11388
- Luebert F, Pliscoff P (2022) The vegetation of Chile and the EcoVeg approach in the context of the International Vegetation Classification project. Vegetation Classification and Survey 3: 15–28. https://doi.org/10.3897/VCS.67893
- MacMahon JA (1988) Warm Deserts. In: Barbour MG, Billings WD (Eds) North American terrestrial vegetation. Cambridge University Press, Cambridge, UK, 231–264.
- Mangen J-M (1993) Ecology and vegetation of Mt.Trikora, New Guinea (Irian Jaya/Indonesia). Travaux Scientifiques du Musée National d’Histoire Naturelle de Luxembourg 21. Minist. des Affaires Culturelles, LU.
- Mares MA [Ed.] (1999) Encyclopedia of the deserts. University of Oklahoma Press, US, 654 pp.
- Miranda F, Hernández E (1963) Los tipos de vegetación de México y su clasificación. Chapingo, MX, 179 pp. https://doi.org/10.17129/botsci.1084
- Miranda F, Sharp AJ (1950) Characteristics of vegetation in certain temperate regions of Mexico. Ecology 31: 313–333. https://doi.org/10.2307/1931489
- Moat J, Smith P [Eds] (2007) Atlas of the vegetation of Madagascar. Kew Publishing. Royal Botanic Gardens, Kew, UK.
- Morrone JJ (2017) Neotropical biogeography: regionation and evolution. CRC Press, Taylor and Francis. https://doi.org/10.1201/b21824
- Mucina L (2019) Biome: evolution of a crucial ecological and biogeographical concept. New Phytologist 222: 97–114. https://doi.org/10.1111/nph.15609
- Mucina L, Laliberté E, Thiele KR, Dodson JR, Harvey J (2014) Chapter 2: biogeography of kwongan: origin, diversity, endemism and vegetation patterns. In: Lambers H (Ed.) Plant life in the sandplains of southwest Australia, a global biodiversity hotspot. University of Western Australia Publishing, Crawley, AU, 35–79.
- Mucina L, Lötter MC, Rutherford MC, van Niekerk A, Macintyre PD, Tsakalos ML, Timberlake J, Adams JB, Riddin T, Mccarthy LK (2021) Forest biomes of South Africa. New Zealand Journal of Botany 60: 377–428. https://doi.org/10.1080/0028825X.2021.1960383
- Mucina L, Rutehrford MC [Eds] (2006) The vegetation of South Africa, Lesotho and Swaziland. Sterlitzia 19. South African National Biodiversity Institute. Pretoria. SA, 807 pp.
- Mueller-Dumbois D, Fosberg FR (1998) Vegetation of the Tropical Pacific islands. Ecological Studies 132. Springer, Cham, CH, 735 pp. https://doi.org/10.1007/978-1-4419-8686-3
- Nakamura Y, Krestov PV (2005) Coniferous forests of thetemperate zone of Asia. In: Andersson F (Ed.) Coniferous forests. Ecosysems of the world 6. Elsevier, Amsterdam, NL, 163–220.
- Nakamura Y, Krestov PV, Omelko AM (2007) Bioclimate and zonal vegetation in Northeast Asia: first approximation to an integrated study. Phytocoenologia 37: 443–470. https://doi.org/10.1127/0340-269X/2007/0037-0443
- Navarro-Sánchez G (2011) Clasificación de la vegetación de Bolivia. Centro de Ecología Difusión Simón I. Patiño, Santa Cruz, BO, 713 pp.
- Navarro-Sánchez G, Molina-Abril JA (2020) The central Andean dry Puna. Characteristics and threat categories. The Encyclopedia of Conservation. Elsevier, Amsterdam, NL.
- Navarro-Sánchez G, Molina-Abril JA (2021) A novel biome concept and classification system based on bioclimate and vegetation – a Neotropical assay. Vegetation Classification and Survey 2: 159–175. https://doi.org/10.3897/VCS/2021/64759
- Navarro-Sánchez G, Molina-Abril JA, Pérez de Molas L (2006) Classification of the forestst of the northern Paraguayan Chaco. Phytocoenologia 36: 473–508. https://doi.org/10.1127/0340-269X/2006/0036-0473
- Oviedo GF (1851–55) [1535] Historia general y natural de las Indias. Real Acad. Historia. Madrid, ES.
- Oyarzábal M, Clavijo J, Oakley L, Biganzoli F, Tognetti P, Barberis I, Maturo HM, Aragón R, Campanello PI, … León RJC (2018) Unidades de vegetación de Argentina. Ecología Austral 28: 40–63. https://doi.org/10.25260/EA.18.28.1.0.399
- Paijmans K [Ed.] (1976) New Guinea vegetation. Australian National University Press, Canberra, AU.
- Peinado M, Aguirre JL, Aparicio A (2017) The Iberian Ranges and Highlands. In: Loidi J (Ed.) The vegetation of the Iberian Peninsula Vol. I. Springer, Cham, CH, 349–512. https://doi.org/10.1007/978-3-319-54784-8_11
- Pew KL, Larsen CPS (2001) GIS analysis of spatial and temporal patterns of human-caused wildfires in the temperate rain forest of Vancouver Island, Canada. Forest Ecology and Management 140: 1–18. https://doi.org/10.1016/S0378-1127(00)00271-1
- Pfadenhauer J, Klötzli F (2014) Die Vegetation der Erde. Grundlage, Ökologie, Verbreitung. Springer, Berlin, Heidelberg, DE, 643 pp. https://doi.org/10.1007/978-3-642-41950-8
- Powers RF, Adams MB, Joslin JD, Fiske JN (2005) Non-Boreal coniferous forests of North America. In: Andersson F (Ed.) Coniferous forests. Ecosysems of the world 6. Elsevier, Amsterdam, NL, 221–292.
- Powers RF, Fiske JN (2005) Western coastal forests. In: Andersson F (Ed.) Coniferous forests. Ecosysems of the world 6. Elsevier, Amsterdam, NL, 246–252.
- Primack RB, Corlett RT (2011) Tropical rain forests. An ecological and biogeographical comparison. 2nd Ed. Wiley-Blackwell, Chichester, UK. https://doi.org/10.1002/9781444392296
- Quézel P (1965) La végétation du Sahara. Du Tchad à la Mauritanie. Gustav Fischer, Stuttgart, DE, 333 pp.
- Quézel P (1995) La flore du basin méditerranéen: origine, mise en place, endémisme. Ecologia Mediterranea 21: 19–39. https://doi.org/10.3406/ecmed.1995.1752
- Quézel P, Barbero M (1982) Definition and characterization of Mediterranean-type ecosystems. Ecologia Mediterranea 8: 15–29. https://doi.org/10.3406/ecmed.1982.1929
- QGIS Development Team (2009) QGIS Geographic Information System. Open Source Geospatial Foundation. http://qgis.org
- R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- Rangel JO, Lowy PD, Aguilar M (1997) Distribución de los tipos de vegetación en las regiones naturales de Colombia. Aproximación inicial. In: Rangel JO, Lowy D, Aguilar M (Eds) Colombia. Diversidad biótica II. Tipos de vegetación en Colombia: Inst. Ci. Nat. (ICN). Universidad Nacional de Colombia, Bogotá, CO, 383–436.
- Read J, Hope G (1996) Ecology of Nothofagus forestst in New Guinea and New Caledonia. In: Veblen TT, Hill RS, Read J (Eds) The ecology and biogeography of Nothofagus forests. Yale University Press, US, 200–256.
- Rivas-Martínez S, Loidi J (1999) Bioclimatology of the Iberian Peninsula. In: Rivas-Martínez . (Eds) Iter Ibericum AD MIM. Itinera Geobotanica 13: 41–47.
- Rivas-Martínez S, Molero J, Marfil JM, Benítez G (2020) Floristic and geobotanical comments concerning Morocco flora. Note I. Naturalia Cantabricae 8 Especial (3): 115–121.
- Rivas-Martínez S, Rivas Sáenz S, Penas A (2011) Worldwide bioclimatic classification system. Global Geobotany 1: 1–643.
- Rizzini CT (1997) Tratado de fitogeografia do Brasil. Aspectos ecológicos, sociológicos e florísticos. Âmbito Cultural Edições Ltda, Rio de Janeiro, BR, 747 pp.
- Rundel PW, Smith AP, Meinzer FC [Eds] (1994) Tropical Alpine environments. Plant form and function. Cambridge University Press, Cambridge, UK, 380 pp. https://doi.org/10.1017/CBO9780511551475
- Rzedowski J (2006) Vegetación de México. 1ª ed, digital. CONABIO, MX, 504 pp.
- Sayer JA, Harcourt CS, Collins NM (1992) The conservation atlas of tropical rain forsts. Africa. IUCN. Macmillan Publishers Ltd, Basingstoke, UK, 287 pp. https://doi.org/10.1007/978-1-349-12961-4
- Schroeder F-G (1998) Lehrbuch der Pflanzengeographie. UTB für Wissenschaft. Quelle and Meyer. Wiesbaden, DE, 457 pp.
- Schultz J (2005) The ecozones of the world. The ecological divisions of the Geosphere. 2nd Ed. Springer, Berlin Heidelberg, DE, 252 pp.
- Sekar KC, Srivastava SK (2010) Rhododendrons in Indian Himalayan region: diversity and conservati on. American Journal of Plant Sciences 1: 131–137. https://doi.org/10.4236/ajps.2010.12017
- Sims PL (1988) Grasslands. In: Barbour MG, Billings WD (Eds) North American terrestrial vegetation. Cambridge University Press, UK, 265–286.
- Smith RIL, Poncet S (1987) Deschampsia antarctica and Colobanthus quitensis in the Terra Firma Islands. British Antarctic Survey Bulletin 74: 31–35.
- Taleb MS, Fennane M (2019) Vascular Plant Communities of Morocco. Phytosociology, Ecology and Geography. Geobotany Studies. Basics, methods and case studies. Springer, Cham, CH, 161 pp. https://doi.org/10.1007/978-3-319-93704-5
- Tallis JH (1991) Plant community history. Long-term changes in plant distribution and diversity. Chapman and Hall, London, UK, 398 pp.
- The Plant List (2013) The Plant List. Version 1.1. http://www.theplantlist.org/1.1/ [Accessed 4 April 2022]
- Timbal J, Bonneau M, Landmann G, Trouvilliez J, Bouhot-Delduc L (2005) European non-Boreal conifer forests. In: Andersson F (Ed.) Coniferous forests. Ecosysems of the world 6. Elsevier, , Amsterdam, NL, 131–162.
- Thornthwaite CW (1933) The climates of the Earth. Geographical Review 23: 433–440. https://doi.org/10.2307/209629
- Tomaselli R (1981) Main physiognomic types and geographic distribution of shrub systems related to Mediterranean climates. In: Di Castri F, Goodall DW (Eds) Ecosystems of the world 11. Mediterranean-type shrublands, Elsevier, Amsterdam, NL, 95–106.
- Troll C (1948) Der asymmetrische Aufbau der Vegetationszonen und Vegetationsstufen auf der Nord- und Südhalbkugel. Berichte des geobotanischen Forschungsinstituts Rübel Zürich, CH, 46–83.
- Vargas Ulate G (1997) La vegetación de América Central: características, transformaciones y protección. Anuario de Estudios Centroamericanos, Universidad de Costa Rica 23: 7–34.
- Walter H (1977) Zonas de vegetación y clima. Ed. Omega. Barcelona, ES, 245 pp.
- Walter H (1979) Vegetation und Klimazonen. 3rd Ed. Ulmer, Stuttgart, DE, 342 pp.
- Walter H (1985) Vegetation of the Earth and Ecological Systems of the Geobiosphere. 3rd Ed. Springer-Verlag, Belin, DE, 319 pp. https://doi.org/10.1007/978-3-662-02437-9_3
- Wardle P (1991) Vegetation of New Zealand. Blackburn Press. Caldwell, N. J., US, 672 pp.
- Weber MG, van Cleve K (2005) The boreal forests of North America. In: Andersson F (Ed.) Coniferous forests. Ecosysems of the world 6. Elsevier, Amsterdam, NL, 101–130.
- Wesche K, Ambarli D, Kamp J, Török P, Treiber J, Dengler J (2016) The Palaearctic steppe biome: a new synthesis. Biodiversity and Conservation 25: 2197–2231. https://doi.org/10.1007/s10531-016-1214-7
- West NE (1988) Intermountain deserts, shrub steppes, and woodlands. In: Barbour MG, Billings WD (Eds) North American terrestrial vegetation. Cambridge University Press, Cambridge, UK, 209–230.
- White F (1983) The vegetation of Africa. Natural Resources Research XX. UNESCO, 356 pp.
- Whittaker RH (1970) Communities and ecosystems. Mac Millan Co. London, UK, 158 pp.
- Yong-Chang S, Lian-Jun D (2016) Evergreen Broad-Leaved Forest of East Asia. In: Box E (Ed.) Vegetation Structure and Function at Multiple Spatial, Temporal and Conceptual Scales. Geobotany Studies. Springer, Cham, CH, 101–128. https://doi.org/10.1007/978-3-319-21452-8_3
- Zohary M (1973) Geobotanical foundations of the Middle East. Vol. 2. Gustav Fischer, Stuttgart, DE, 683 pp.
E-mail and ORCID
- Javier Loidi (Corresponding author, javier.loidi@ehu.eus), ORCID: https://orcid.org/0000-0003-3163-2409
- Gonzalo Navarro-Sánchez (gonzalonavarrosanchez@gmail.com), ORCID: https://orcid.org/0000-0001-9890-5112
- Denys Vynokurov (denys.vynokurov@gmail.com), ORCID: https://orcid.org/0000-0001-7003-6680
Supplementary materials
table with the climatic data of the 616 selected locations.
Supplementary maps and figures. S3.1 Importance of the selected variables in the PCA ordination axis 1 vs. 3; S3.2. Biomes and Subbiomes of Africa S3.3. Biomes and Subbiomes of Asia; S3.4. Biomes and Subbiomes of Australasia; S3.5. Biomes and Subbiomes of Europe S3.6. Biomes and Subbiomes of North America; S3.7. Biomes and Subbiomes of South America; S3.8. World distribution of ombrotypes; S3.9. World distribution of Io.
Boxplots of the selected parameters for the domains, ecozones, biomes and subbiomes.